Application Brief
This is an Application Brief and does not contain a detailed Experimental section.
TG Rosano1,2, JM Rumberger1, RM Konetchy Jr.1, KL Scholz1, M Wood1,3
1 National Toxicology Center, United States
2 Department of Pathology and Laboratory Medicine, Albany Medical College, United States
3 Waters Corporation, United Kingdom
Published on May 28, 2025
This is an Application Brief and does not contain a detailed Experimental section.
For forensic toxicology use only.
Xylazine is a non-opioid central nervous system (CNS) depressant that is authorized for veterinary use in the USA as an anesthetic sedative and muscle-relaxant analgesic. Increasing human misuse of xylazine has been reported in a forensic setting, including opioid-related overdoses and drugs in driving casework. Xylazine has also been detected as an adulterant in seized drug samples with evidence that its use is spreading geographically. Expanded toxicology screening for xylazine use is therefore needed in forensic practice. This application brief reports on an existing direct-to-definitive toxicology screen panel with quantitative monitoring for xylazine and its major metabolite in urine. The brief summarizes a previously reported definitive ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method for a panel of 102 toxicologically relevant analytes and details the recent update and validation to include xylazine and 4-hydroxy xylazine.
A review of xylazine prevalence in forensic casework shows an expanding involvement of xylazine in drug-related fatalities and impaired driving.1,2 Xylazine adulteration of illicit drugs, including heroin and fentanyl (‘tranq-dope’), is purported to potentiate and prolong the effects of the opioids, leading to enhanced risk of fatal overdose. Public health studies point to enhanced danger of adverse outcomes from street drugs containing xylazine.3 In April 2023, the Director of the Office of National Drug Control Policy in the USA designated fentanyl adulterated with, or associated with, xylazine as an emerging drug threat.4 The prevalence of xylazine in combination with opioids is also being reported in other countries,5–8 and it is likely that prevalence reports may be underestimated due to a lack of availability of toxicology screens for xylazine in most forensic laboratories.
A novel UHPLC-MS/MS method has previously been reported and is currently used in the laboratory for direct-to-definitive detection and quantification of 102 drugs and drug metabolites in urine.9 The method’s novelty involves use of a matrix normalization technique called Threshold Accurate Calibration, or TAC. There is growing awareness among analysts using liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening of large multi-drug and metabolite panels that urine matrix variability may significantly affect detection and quantitation accuracy. The best practice approach has historically been the use of a deuterated isotope derivative of each drug or metabolite as an internal standard, but analyte-specific internal standard availability and cost have limited this approach in large testing panels. Analytical conditions and validation results are presented here for expansion of a TAC-based LC-MS/MS method to include xylazine and 4-hydroxy xylazine identification and quantitation. Preliminary casework findings are also presented.
Xylazine and 4-hydroxy xylazine were from Cayman Chemical (Ann Arbor, MI, USA). Injection recovery standard (methapyrilene) was from Cerilliant (Round Rock, TX, USA). All other reference analytes in the previously described method9 were either from Cerilliant (MilliporeSigma, Burlington, MA, USA), LGC, or Lipomed, (Cambridge, MA, USA). Purified beta glucuronidase (IMCSzyme, >50,000units/mL) and buffer were from Integrated Micro Chromatography Systems (Columbia, SC, USA).
Multi-analyte spike: A mixed multi-analyte spike reagent was prepared at 10 times the upper limit of quantification (ULOQ) of each analyte; for xylazine and its metabolite, this was 10,000 ng/mL.
Hydrolysis and recovery control reagent: Methapyrilene (used as a control for injection precision) and beta glucuronidase (for hydrolysis) were diluted in hydrolysis buffer to give a final concentration of 250 ng/mL and >5,000 units enzyme activity, respectively.
Calibrators and quality controls: Analyte negative urine was supplemented with a multi-analyte reference mixture that contained the originally reported 102 analytes plus xylazine and 4-hydroxy xylazine. For the latter, calibrators ranged from 38–500 ng/mL and quality controls were at 38, 200, and 400 ng/mL.
A complete description of the concept of the TAC technique and a step-by-step protocol for the analysis of 102 analytes has been previously published.9 In brief, the technique involves testing of each sample twice, i.e., with, and without, the addition of a known amount of reference analyte. In practice, dual 20 µL aliquots of sample (calibrator, quality control, or case sample) are added to adjacent wells in a 2 mL square 96-well sample collection plate (p/n: 19020641) with 20 µL of water added to each of the ‘Neat’ wells and 20 µL of multi-analyte spike added to the corresponding ‘Spike’ wells. Twenty microliters of hydrolysis and recovery reagent is added to all wells before shaking the plate for 3 min then incubating at 55 °C for 1 hr. Following hydrolysis, 200 µL of starting mobile phase (98% MPA/2% MPB) is added to each well prior to analysis.
Samples were analyzed using a Waters ACQUITY™ UPLC I-Class PLUS System with flow-through-needle (FTN) interfaced with a Waters Xevo™ TQD Mass Spectrometer.
|
UPLC system: |
ACQUITY UPLC I-Class PLUS System (FTN) |
|
Pre-column |
ACQUITY Column In-Line Filter (p/n: 205000343) |
|
Analytical column: |
ACQUITY UPLC BEH™ Phenyl Column, 1.7 µm, 2.1 x 50 mm |
|
Column temperature: |
45 °C |
|
Sample temperature: |
25 °C |
|
Mobile phase A (MPA): |
2 mM ammonium formate in water |
|
Mobile phase B (MPB): |
2 mM ammonium formate in methanol, 0.1% formic acid |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
5 µL |
The solvent gradient was as follows: 2% MPB (0–0.5 min), 2–70% MPB (0.5–2.2 min), 70–90% MPB (2.2–2.7 min), and 90–2% MPB (2.7–3.0 min), hold at 2% MPB for 0.3 min for re-equilibration.
|
MS system: |
Waters Xevo TQD Mass Spectrometer |
|
Ionization mode: |
ESI Positive |
|
Capillary voltage: |
0.55 kV |
|
Desolvation gas: |
800 L/hr |
|
Desolvation temperature: |
550 °C |
|
Source temperature: |
150 °C |
|
MRM parameters: |
See table |
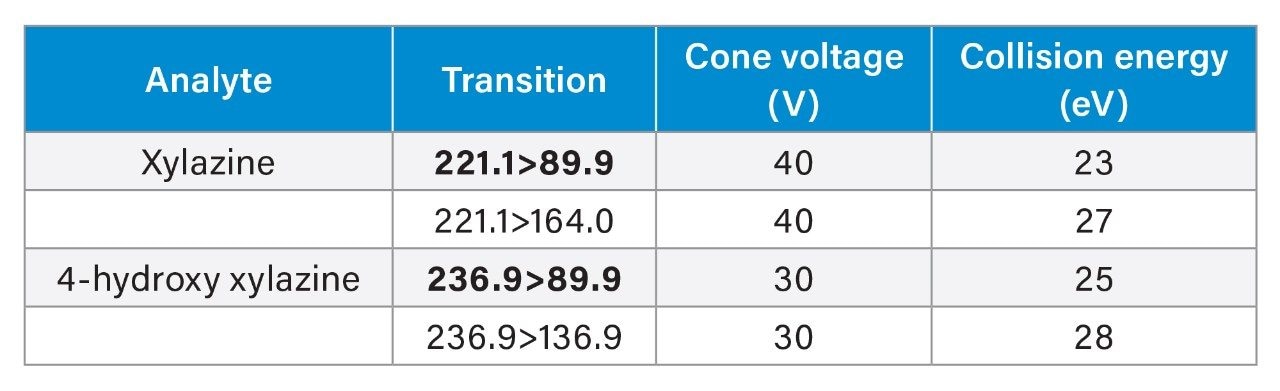
Data were acquired and analyzed using MassLynx™ Software (V4.1). Quantification was performed using TargetLynx™ XS Software and then exported into a laboratory-developed spreadsheet template for evaluation of ion response and acceptance criteria for all samples. The template also automates calculation of the * TAC ratio according to the formula below and plots the calibration curve.
* TAC ratio = ‘Neat’ peak-area response/(’Spike’ peak-area response – ‘Neat’ peak-area response)
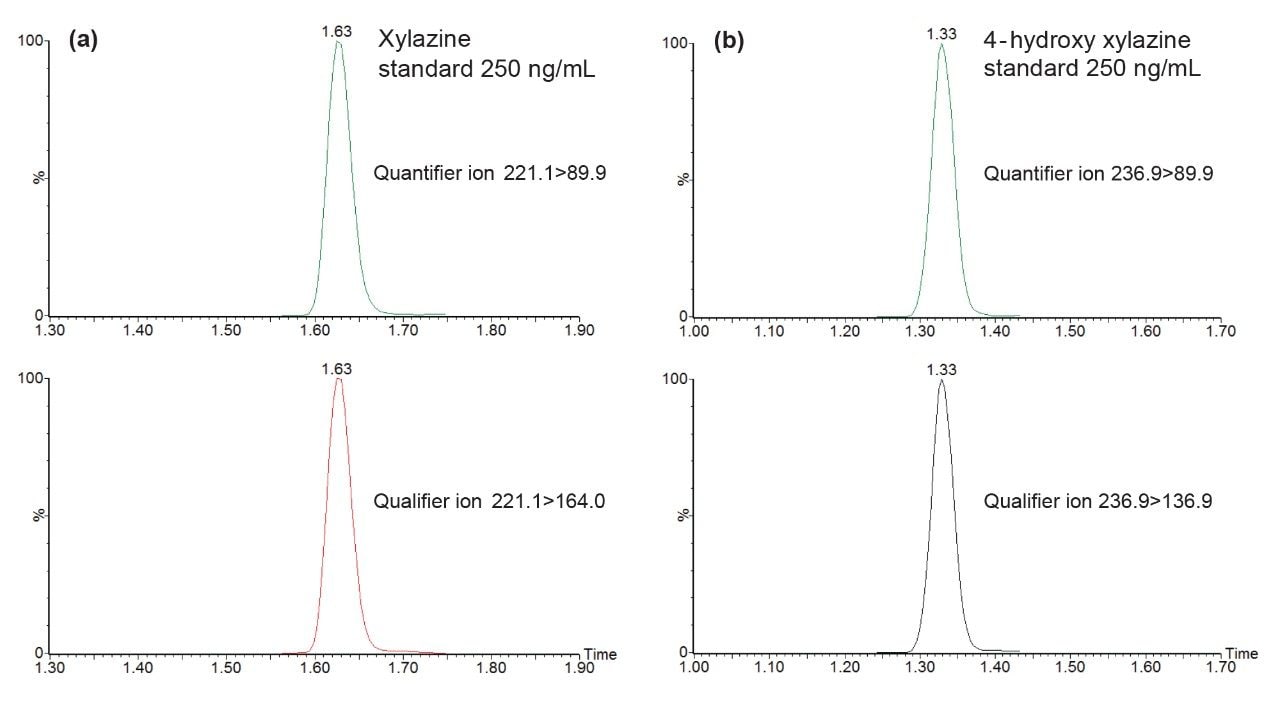
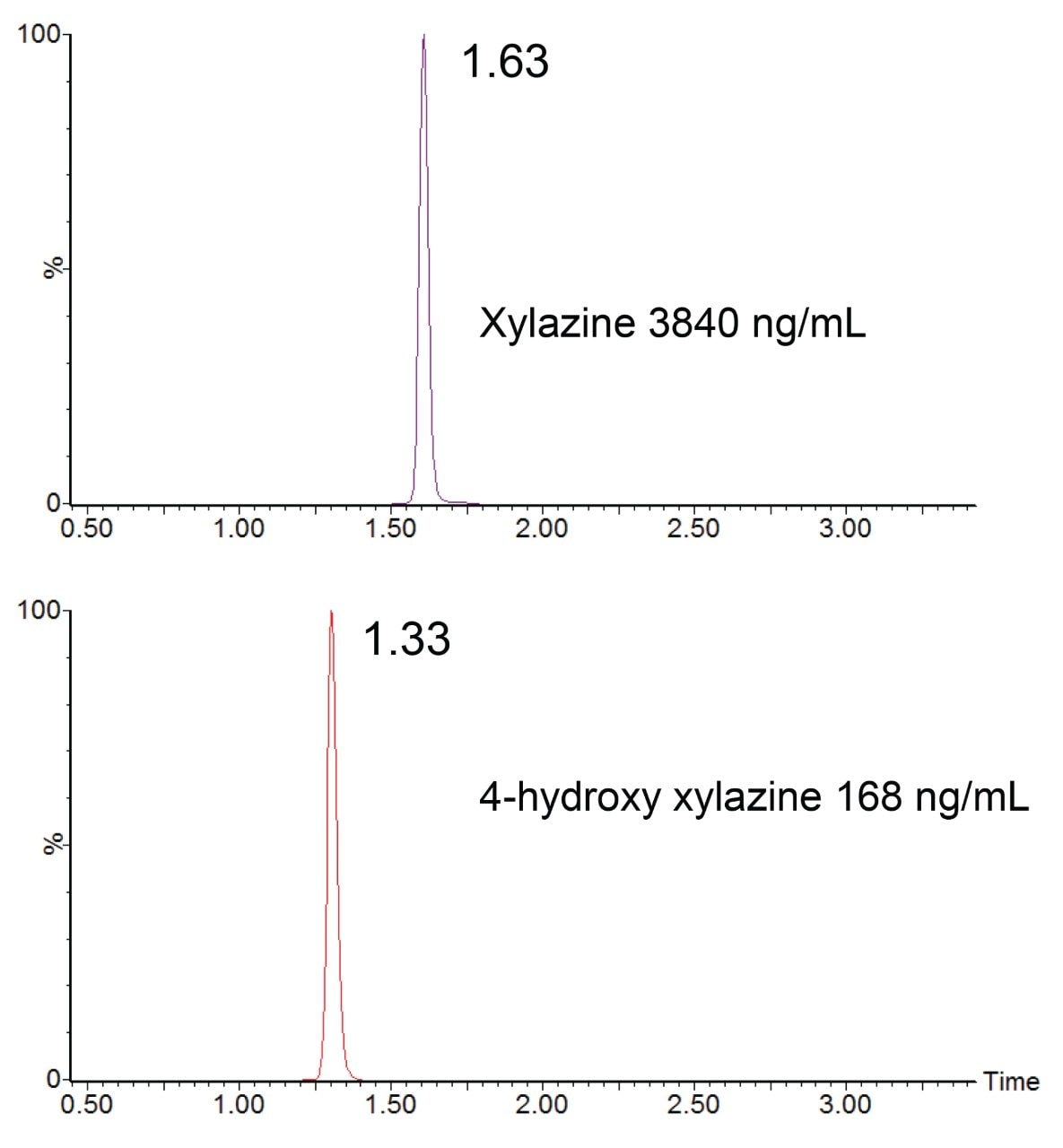
The aim of the present study was to apply the published protocol9 in the update and validation of new analytes, xylazine, and its major urinary metabolite 4-hydroxy xylazine. Multiple reaction monitoring (MRM) conditions were determined for both analytes by infusion of individual solutions of reference material. Details of the resulting instrument and ion acquisition parameters are listed in the MRM parameters table. The MRM conditions were updated in the analysis method and analysis revealed efficient chromatographic peaks with retention times of 1.63 min for xylazine and 1.33 min for 4-hydroxy xylazine as shown in Figures 1 (a) and (b) MRM analysis for the additional 102 analytes was also acquired in the analysis (MRM chromatograms are not shown).
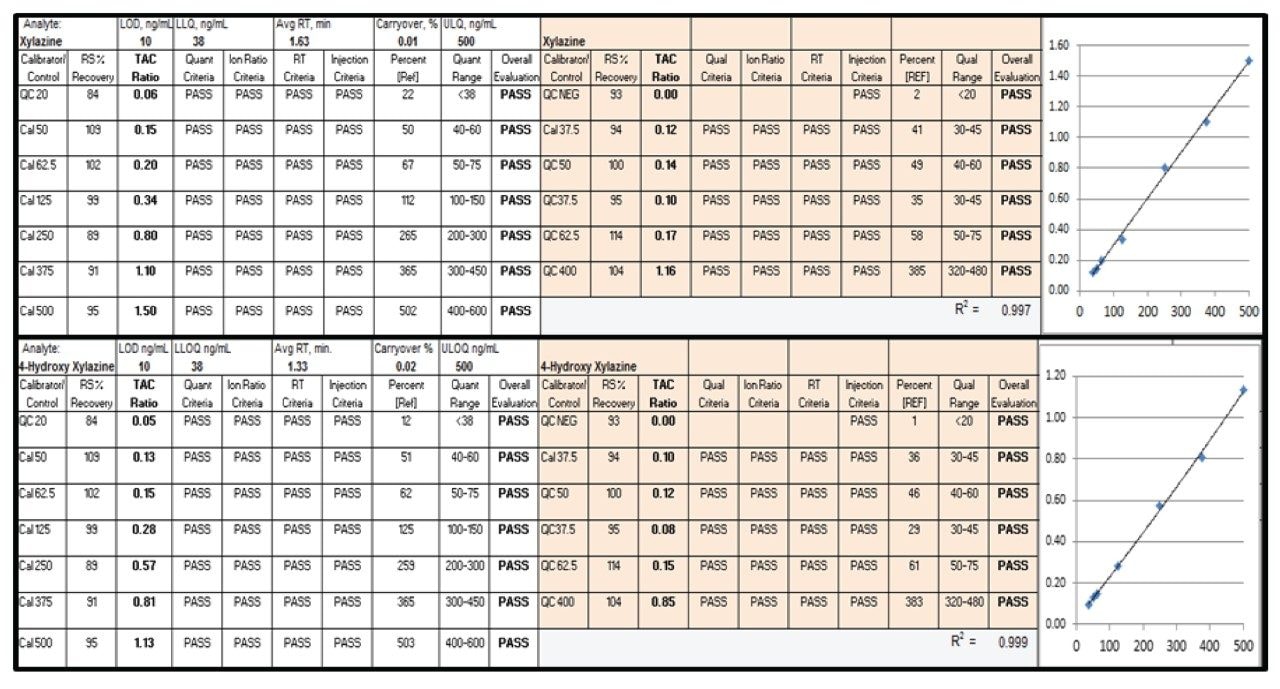
Calibrator and quality control data handling for the TAC approach involves analyte-oriented data analysis using criteria-based evaluation of chromatographic and MS/MS performance acceptability. Figure 2 shows an example of data handling for xylazine and 4-hydroxy xylazine, including calibration plots. Acceptance-criteria performance for analyte concentration, transition ion ratio, chromatographic retention time, injection recovery, and calibration are all displayed in a format that allows optimum ease in data review by the certifying scientist. Similar data for the 102 other analytes is not shown.
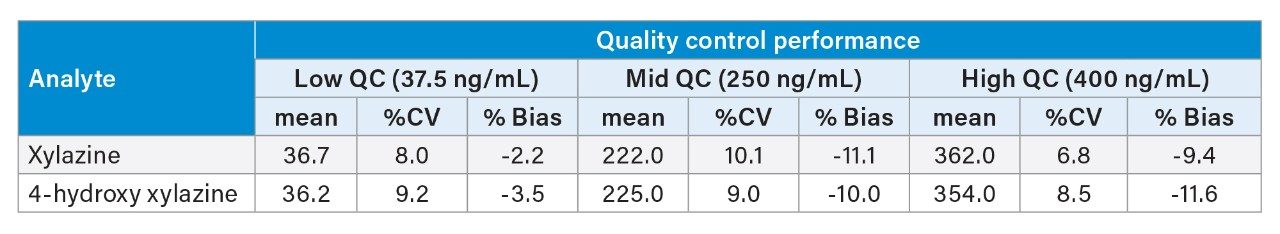
Analytical performance was validated using American National Standards Institute/Academy Standards Board (ANSI/ASB) guidelines for forensic drug testing and New York State laboratory standards for clinical toxicology.10,11 Precision and bias studies are shown in Table 1. Average percent coefficient of variation ranged from 6.8–10.1% with a bias ranging from -11.5 to -2.2%. Calibration model validation involved calibration performance studied over five analytical runs with R2 ranging from 0.994–0.999 and 0.998–1.000 for xylazine and 4-hydroxy xylazine, respectively, and with calibrators within +20% of target concentration for all runs. Carryover assessment in negative samples analyzed after an ULOQ calibrator in multiple analytical runs averaged 2.7% for xylazine and 2.9% for 4-hydroxy xylazine. Matrix effect studies revealed consistent ion enhancement in nine urine matrices with xylazine supplemented at 100 ng/mL (16% ion effect) and 800 ng/mL (7%) concentrations and for 4-hydroxy xylazine at 100 ng/mL (18%) and 800 ng/mL (13%) concentrations, with variability in ion effects (%CV) ranging from 10–13% across all studies. Analytical specificity was validated in ten analyte negative urine analyses and with 124 commonly tested drugs and metabolites tested in the laboratory’s blind sample proficiency testing program. The limit of detection (LOD) was validated by discrimination of TAC ratio in analyte-negative urines with and without xylazine and 4-hydroxy xylazine supplementation. Nine analyte-negative urine pools were tested in triplicate with and without supplementation at the LOD concentration (10 ng/mL) for both analytes. The mean LOD TAC ratio response (xylazine 0.0260: 4-hydroxy xylazine 0.0236) exceeded the mean +3.3SD TAC ratio of the negative sample analyses (xylazine 0.0011: 4-hydroxy xylazine 0.0007).
Process stability was assessed by repeat analysis of four control pools (A-D) at 18 hours after the initial analysis time (0 hours). Analyte recovery for xylazine (90–111%) and 4-hydroxy xylazine (91–103%) was determined with %CVs of 11.2% and 6.0%, respectively, meeting the criteria for process stability. Dilution integrity was also assessed using urine control pools containing 900 ng/mL of xylazine and 4-hydroxy xylazine, with dilutions in analyte-negative urine of 1-in-2, 1-in-5 and 1-in-10. Dilution recoveries were within 20% of target concentration for all control pools.
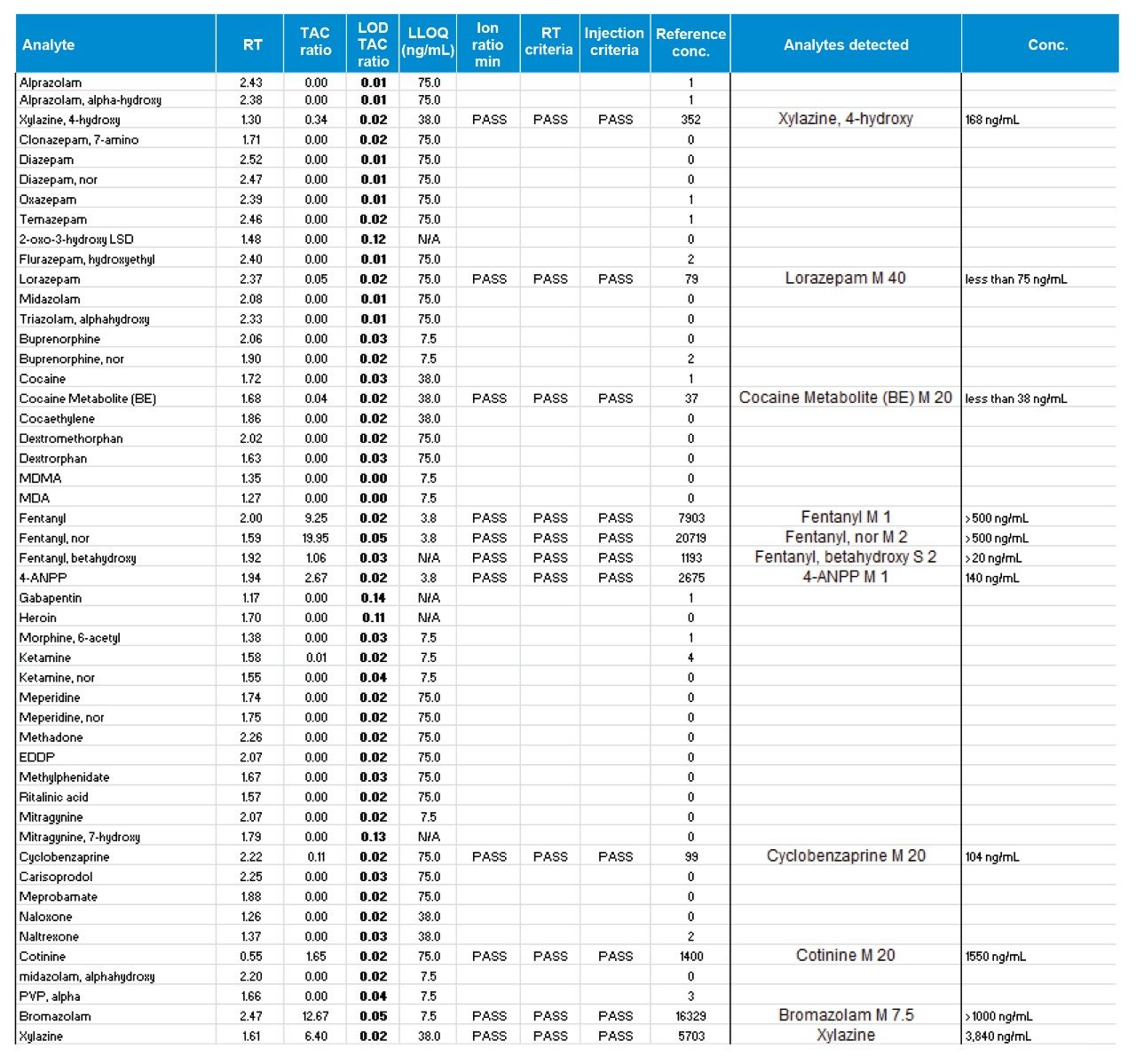
Data handling for case samples was performed by evaluation of all requested analytes together, as shown in Figure 3. The display shows data and results for a xylazine-positive case which was also positive for illicit fentanyl and a number of other drugs. The TAC ratio for each analyte is shown along with the TAC ratio of the LOD control sample. If the TAC ratio in the case exceeds the LOD and also meets criteria for ion ratio, retention time, and injection recovery, then the analyte is listed as detected with a calculated concentration.
Acceptance of case finding identified in the data reporting requires a certifying scientist’s review of the MS/MS acquisition data, along with the total ion chromatograms (TIC) as shown for xylazine and 4-hydroxy xylazine in Figure 3, which also displays analyte concentrations and retention times.
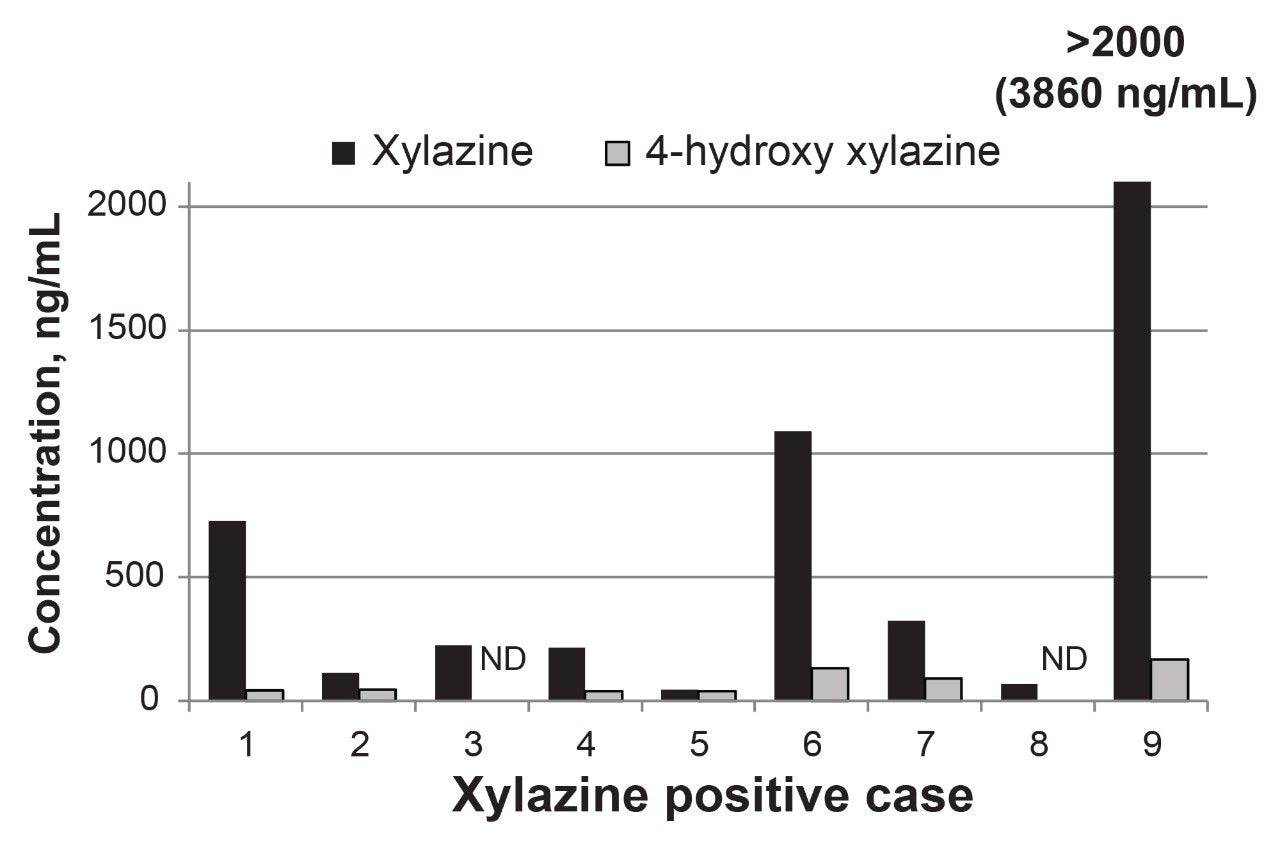
A review of post-validation analyses for an initial cohort of xylazine positive forensic cases showed xylazine and 4-hydroxy xylazine concentration ranges of 58–3860 ng/mL and <38–168 ng/mL, respectively. Urinary concentration of xylazine consistently exceeded 4-hydroxyxylazine as shown for the individual case in Figure 4. Testing with and without enzymatic hydrolysis of glucuronide conjugates revealed the significant of 4-hydroxy xylazine glucuronidation in renal clearance (45–63%) and the importance of the hydrolysis step. As expected, glucuronidation of the non-hydroxylated parent drug was not detected. Xylazine adulteration of fentanyl was evident in each of the cases with concomitant fentanyl and norfentanyl concentrations ranging from 260 ng/mL to greater than 500 ng/mL. Other drugs were also found in the xylazine-positive cases, including gabapentin, tramadol, bromazolam, lorazepam, cyclobenzaprine, cotinine, cocaine metabolite (benzoylecgonine), morphine, heroin metabolite (6-acetylmorphine), and methamphetamine.
The urinary toxicology screen has been updated and validated to include xylazine and 4-hydroxy xylazine. Many of the calibration and quality control validation studies were conducted as part of the routine analytical runs, prior to implementation of testing in casework samples. Initial application to routine casework has confirmed the presence of xylazine use within the region and has demonstrated the relative detection sensitivity of urine xylazine and 4-hydroxy xylazine testing. While this laboratory uses the novel TAC approach to normalize matrix effects in UHPLC-MS/MS analysis, the method may also be applied in modifications employing traditional stable isotope internal standardization.
720008798, May 2025