Assessment of Biosimilarity in mAb-based Therapeutics Using SEC-MALS
Bretton Fletcher, Sophia Kenrick
Waters | Wyatt Technology™, United States
Published on December 19, 2025
Abstract
The development and approval of biosimilar monoclonal antibody (mAb) therapeutics offers a promising path to reducing healthcare costs and increasing patient access to life-saving treatments. However, demonstrating biosimilarity requires rigorous analytical characterization of both the candidate and reference products. This application note highlights how size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS) provides a simple, robust, fit-for-purpose workflow to assess critical quality attributes (CQAs), such as purity, aggregation, fragmentation, and self-association behavior. Using SEC-MALS to compare two mAb reference products (AVASTIN® and REMICADE®) to their respective biosimilar counterparts, Zirabev™ and Avsola®, illustrates the power of SEC-MALS to reveal subtle but often important differences in biophysical properties that may not have been apparent from SEC-UV alone.
Benefits
- Multiple CQAs were measured simultaneously for four commercially available mAb-based therapeutics, demonstrating the power of SEC-MALS in addressing regulatory requirements for biosimilarity studies.
- Molar mass, mass fraction of each species, UV extinction coefficients, and other biophysical attributes were measured in a single SEC-MALS workflow, enabling the identification of each eluting species and providing a robust quantitative assessment of sameness between candidate and reference mAb-based therapeutics.
- MALS measurements uncovered concentration-dependent protein-protein interactions that would not have been detected with traditional SEC-UV alone, providing key insights into protein stability, aggregation, and self-association, qualities that may help strengthen the case for biosimilarity.
Introduction
Monoclonal antibodies have become a cornerstone of modern biotherapeutics, with applications in oncology, autoimmune diseases, and beyond. As patents for innovator biologics expire, biosimilars are emerging as cost-effective alternatives. Regulatory agencies, such as the FDA and EMA, require comprehensive analytical comparability studies to establish biosimilarity—encompassing primary structure, post-translational modifications, higher-order structure, and impurity profiles.
While SEC-UV is commonly used to assess size variants, its reliance on column calibration does not allow for absolute size or molar mass measurements. SEC-MALS overcomes the limitations of traditional SEC-UV by providing direct molar mass measurements across the chromatogram without needing to assume that the analyte elutes in accordance with a calibration curve. Combined with differential refractive index (dRI) and UV detection, SEC-MALS enables multi-attribute quantification (MAQ) of key biophysical properties in a single, streamlined workflow.
Here, a typical UHPLC SEC-MALS setup is used to perform measurements on four different commercially available mAb-based drug products, assessing multiple CQAs in one simple workflow and demonstrating the ability of multi-angle light scattering to reveal transient protein-protein interactions that would otherwise be missed with other techniques.
Experimental
Monoclonal antibody samples were analyzed using an ACQUITY™ Premier UPLC™ System coupled to a microDAWN™ multi-angle light scattering Photometer, microOptilab™ differential Refractometer, and ACQUITY Premier PDA eλ Detector collecting UV absorbance at 280 nm. Separation was performed using a Waters ACQUITY Premier Protein Column (250 Å, 1.7 µm, 4.6 x 150 mm) with a phosphate-buffered saline (PBS; 50 mM sodium phosphate, 50 mM sodium chloride, pH 6.7) mobile phase. Data were processed using ASTRA™ Software.
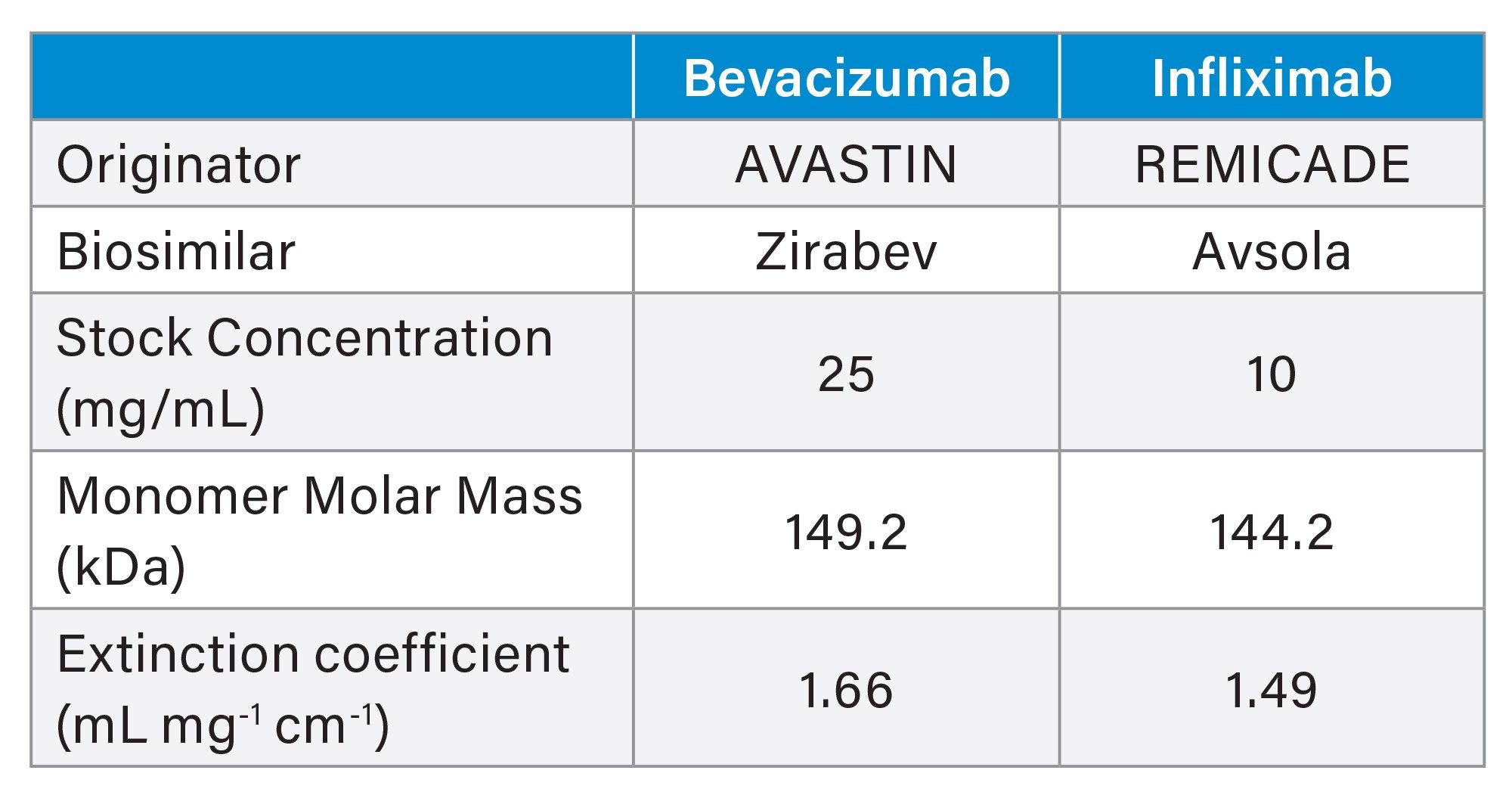
Table 1 summarizes the samples analyzed by SEC-MALS. All samples were ordered from Besse Medical and stored and prepared in accordance with the prescribing information for each commercial product. AVASTIN and Zirabev were purchased as single-dose vials and used as-is, at 25 mg/mL. REMICADE and Avsola were purchased as lyophilized powder and reconstituted in 10 mL of water for a final concentration of 10 mg/mL.
LC Conditions
|
LC system: |
ACQUITY Premier UPLC System |
|
Detection: |
microDAWN multi-angle light scattering Detector microOptilab differential Refractometer ACQUITY Premier PDA eλ Detector, collecting UV absorbance at 280 nm |
|
Vials: |
Waters Clear Glass 12 x 32 mm Screw Neck Total Recovery Vials |
|
Column(s): |
ACQUITY Premier Protein Column (250 Å, 1.7 µm, 4.6 x 150 mm) |
|
Column temperature: |
25 °C |
|
Sample temperature: |
6 °C |
|
Injection volume: |
0.1–3 µL (AVASTIN, Zirabev); 0.25-7.5 µL (REMICADE, Avsola) |
|
Flow rate: |
0.35 mL/min |
|
Mobile phase: |
PBS; 50 mM sodium phosphate, 50 mM sodium chloride, pH 6.7 |
Data Management
|
Chromatography control: |
HPLC CONNECT™ 4.0 Software |
|
Data collection and analysis: |
ASTRA 8.3 Software |
Results and Discussion
Bevacizumab: Comprehensive Biophysical Characterization
SEC-MALS provided multiple CQAs for two commercial bevacizumab drug formulations. Figure 1 shows UV chromatograms for the two samples. The main monomer peak for each drug product is well-separated from low molecular weight species (LMWS) and two high molecular weight species (HMWS). Both the weight-averaged molar mass (Mw) and measured UV extinction coefficient (ε) for the monomer mAb peaks provide key insights and a robust assessment of biosimilarity. SEC-MALS analysis of the reference and biosimilar drug show good agreement between the measured monomer molar masses and extinction coefficient and the expected values based on the amino acid sequence (Table 2). These data showcase the accuracy of SEC-UV-MALS-dRI measurements and the use for MALS in biophysical characterization of mAbs while leveraging the same simple workflow that would be used for traditional SEC-UV analysis.
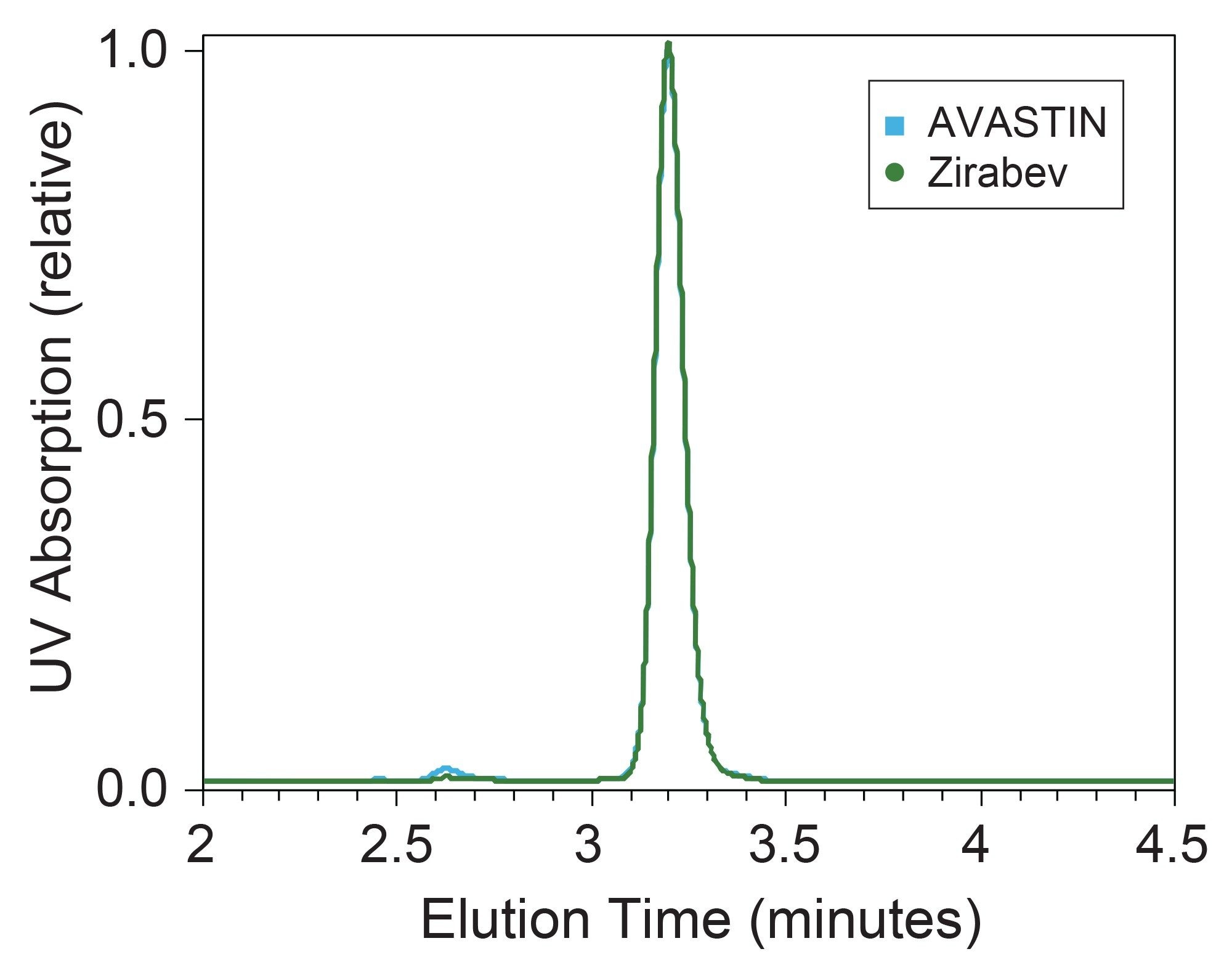
Additionally, the online concentration detectors enable the measurement of mass recovery or the total amount of mAb that elutes off the column. Here, a mass recovery for AVASTIN and Zirabev of 98.3% and 97.5% (standard deviation less than 0.5) is observed, respectively, providing confidence in the chromatographic method while simultaneously enabling active monitoring of system health and performance. These measurements make an SEC-MALS workflow not only good for biophysical characterization of analytes but also useful as a QC tool for assessing instrumentation over time.
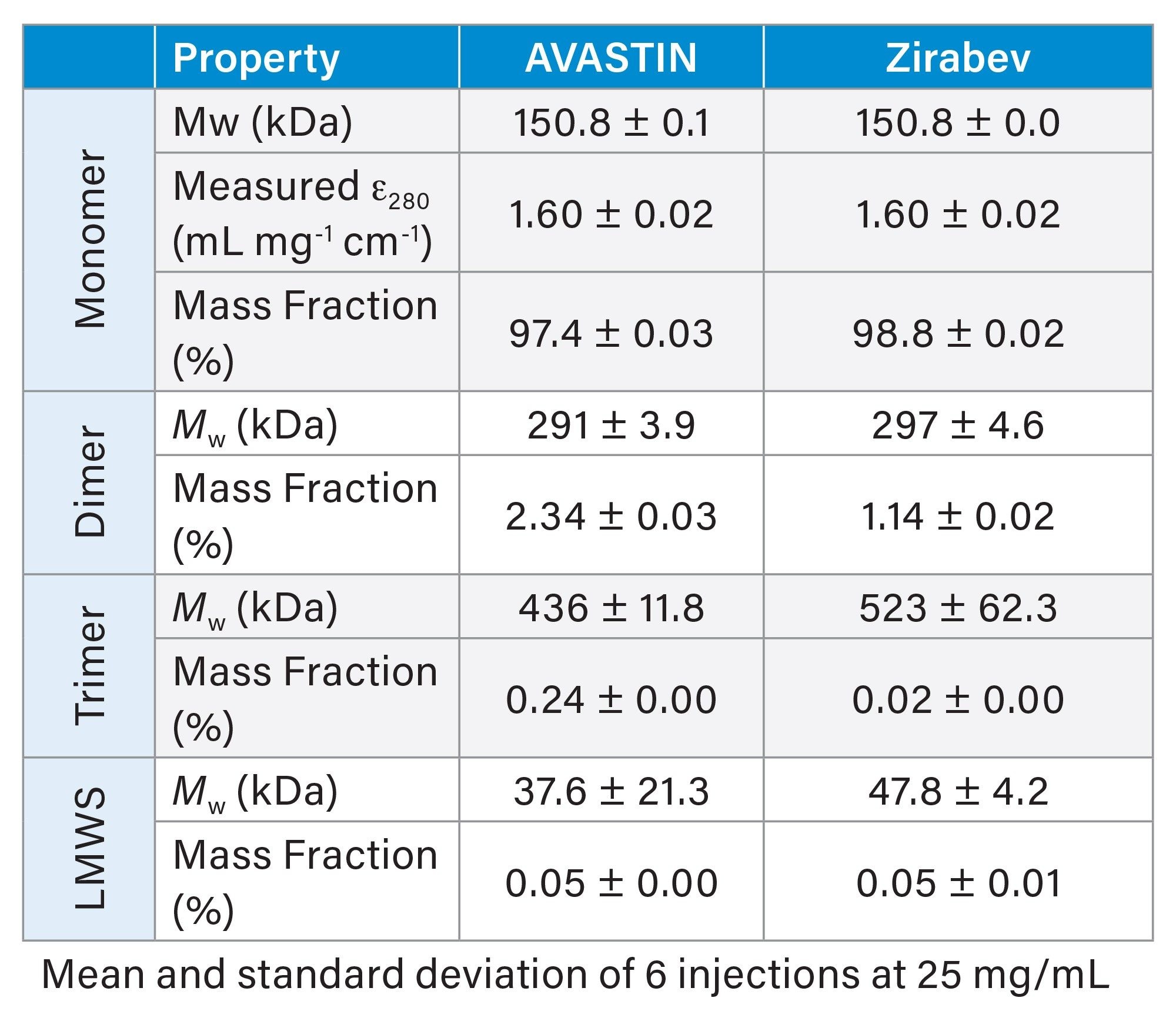
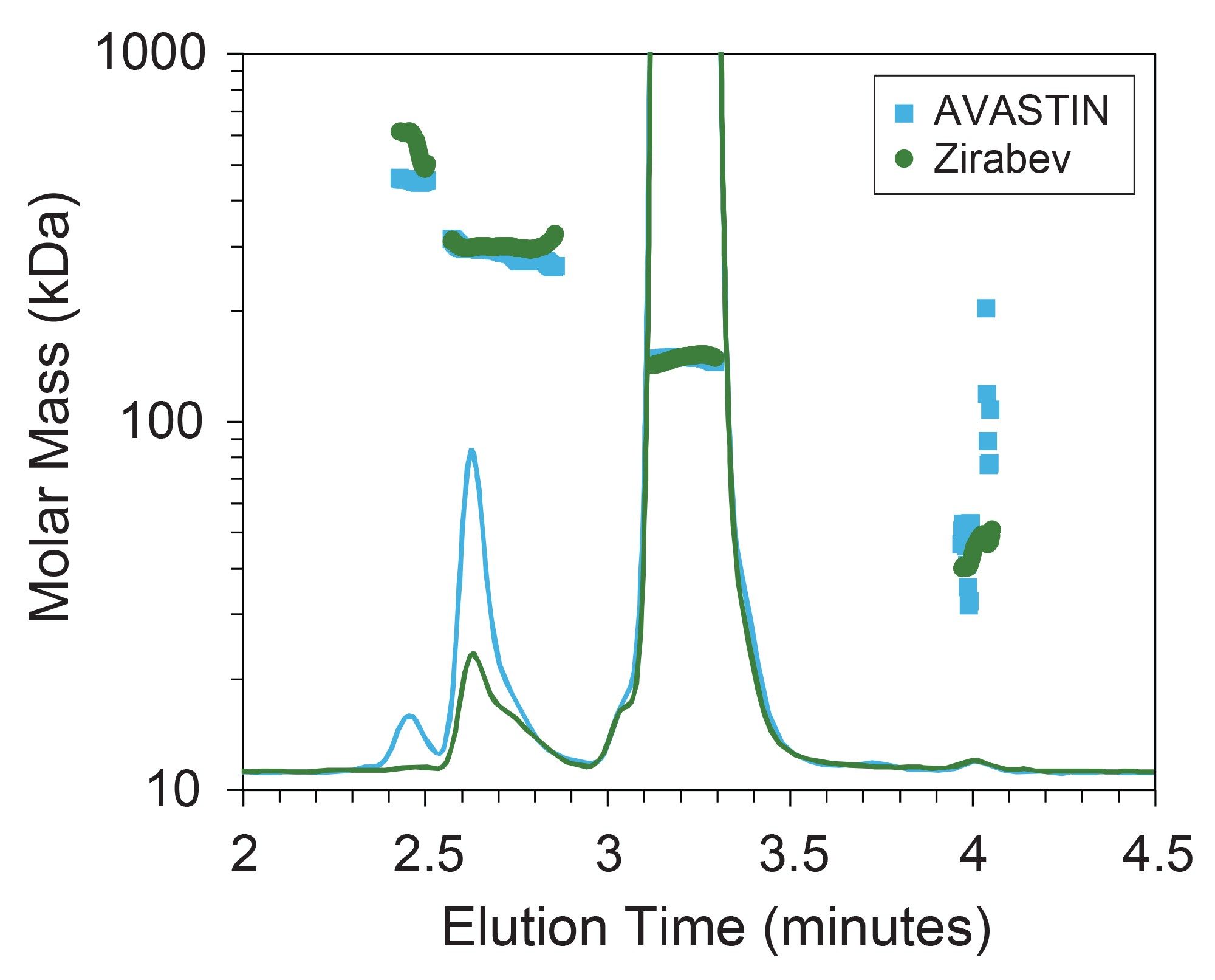
While the identities of the main eluting species for each drug product appear nearly identical when comparing the measured Mw and ε, differences in the mass fractions of each eluting species (Table 2) suggest non-identical aggregation behavior in the two formulations. For both samples, Mw measured for two HMWS corresponded to mAb dimer and trimer, respectively. However, the mass fraction of higher molecular weight species is higher for AVASTIN than for Zirabev. Figure 2 shows the same UV chromatograms as seen in Figure 1, zoomed in on the relevant elution region and overlaid with slice-by-slice molar mass measurements for each sample. While the difference in UV peak area is apparent from the chromatogram alone, the addition of MALS enables the comparison of absolute molar mass between samples despite the apparent differences in sample composition.
Infliximab: SEC-MALS Reveals Reversible Self-association
These experiments were repeated with infliximab drug products REMICADE and Avsola, with the injection volume adjusted to ensure the same total mass (25 µg) was injected for both the bevacuzimab and infliximab samples. Mass recovery measured across the entire elution range appears to be lower for Avsola (89.2% ± 0.2) and REMICADE (92.1% ± 0.2) than for AVASTIN and Zirabev but still in alignment with intended injection amounts. Surprisingly, the chromatograms for REMICADE and Avsola exhibited significant peak asymmetry compared to AVASTIN and Zirabev, despite the similarity in expected molar mass, size, and conformation of the monomeric mAbs.
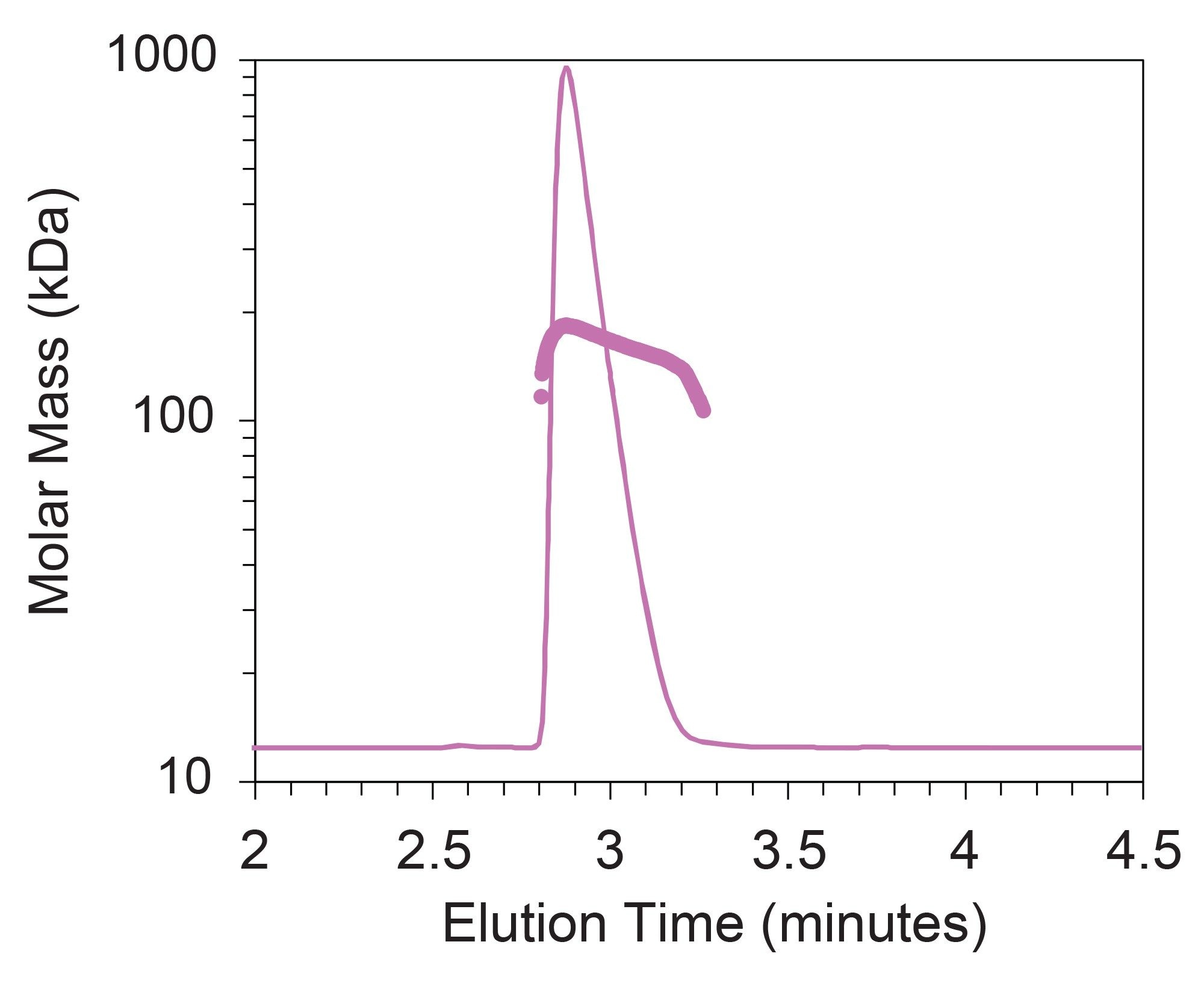
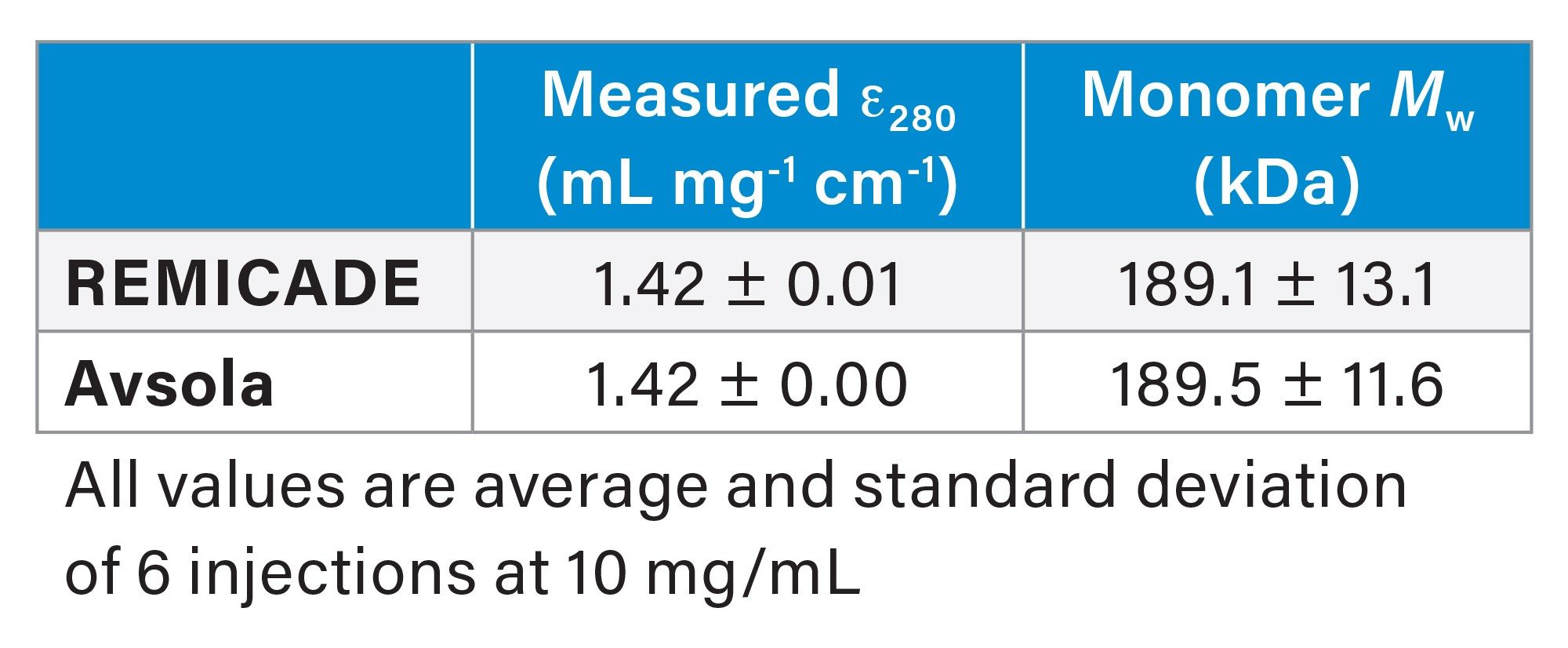
While measured UV extinction coefficients matched expected values quite well, Mw measured across the main peak is higher than expected for both REMICADE and Avsola (Table 3). A closer look at the slice-by-slice molar mass data for the infliximab samples also reveals heterogeneity across the peak, contrary to the expected monodispersity of a purified monomeric protein. Specifically, molar mass is highest at the apex of the elution peak, where the concentration of mAb would be highest as it elutes from the column.
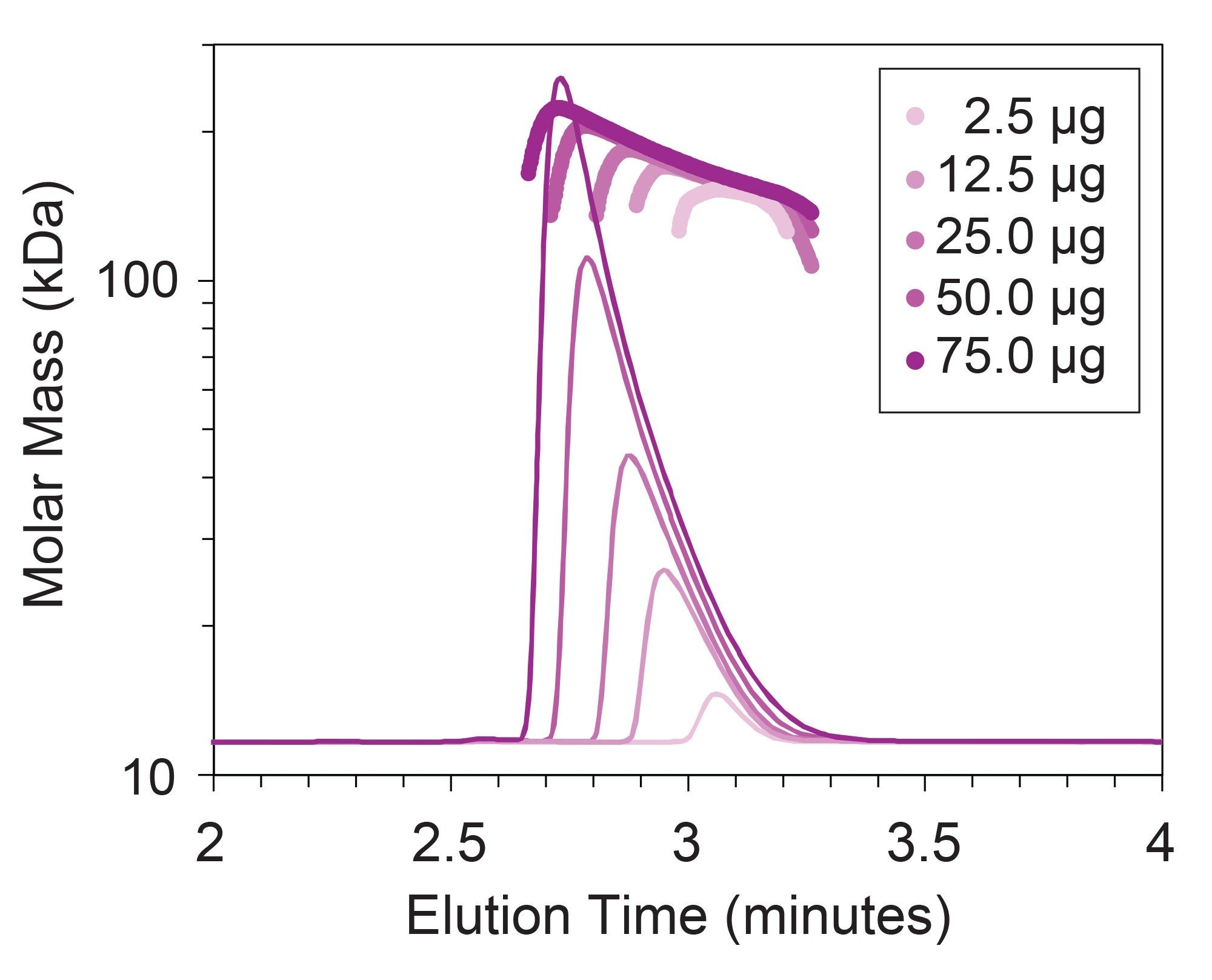
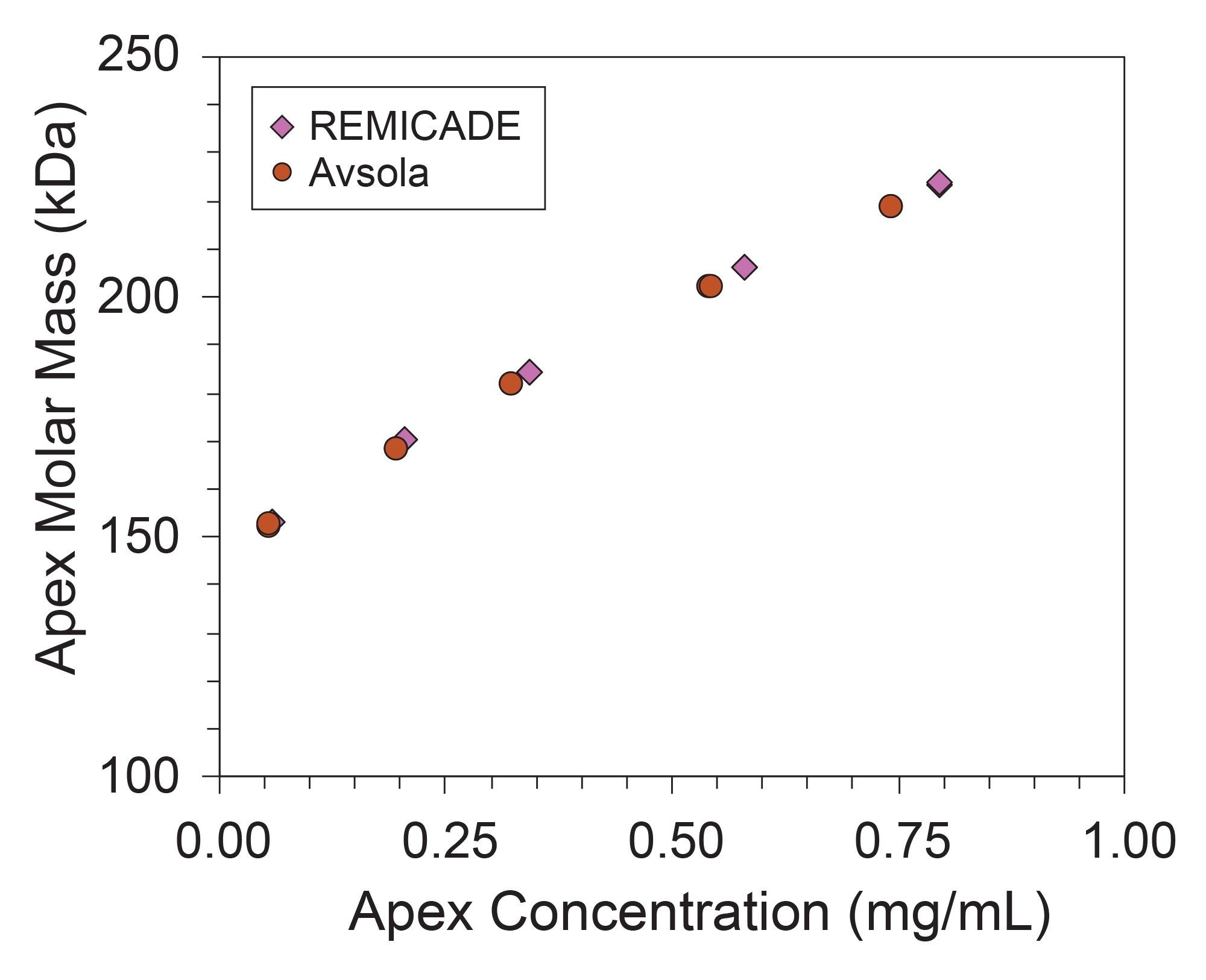
This trend in molar mass along the elution profile implies the possibility of concentration-dependent reversible interactions among mAbs. To investigate the concentration-dependent nature of these reversible interactions, varying amounts of mAb from 2.5 µg to 75 µg were injected to vary the eluting mAb concentration. The chromatograms and molar mass profiles for these injections are plotted in Figure 4, where the trend in concentration-dependent apex molar mass (Mp) is made even clearer. That data is further summarized in Figure 5.
The concentration-dependent increase in molar mass can provide insight into the affinity and stoichiometry of the interaction.2 While these results demonstrate the ability of SEC-MALS to reveal these interactions, orthogonal light scattering techniques, such as composition-gradient multi-angle light scattering (CG-MALS), can provide more detailed characterization of the equilibrium association and mechanism of the interaction, and may also provide additional information on biosimilarity.3–5
Conclusion
SEC-MALS provides a powerful, intuitive platform for assessing biosimilarity of mAb therapeutics. By enabling direct measurement of molar mass, extinction coefficients, and mass fractions of size variants, SEC-MALS reveals critical insights into higher-order structure and stability that may be missed by conventional SEC-UV. The case studies presented here demonstrate how a single, streamlined workflow can support regulatory requirements for biosimilarity assessment and serve as a valuable tool for formulation development, quality control, and comparability studies.
References
- Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- Das, S., Stivison, E., Folta-Stogniew, E. & Oliver, D. Reexamination of the Role of the Amino Terminus of SecA in Promoting Its Dimerization and Functional State. J. Bacteriol. 190, 7302–7307 (2008).
- Esfandiary, R., Parupudi, A., Casas-Finet, J., Gadre, D. & Sathish, H. Mechanism of Reversible Self-Association of a Monoclonal Antibody: Role of Electrostatic and Hydrophobic Interactions. J. Pharm. Sci. 104, 577–586 (2015).
- Some, D., Pollastrini, J. & Cao, S. Characterizing Reversible Protein Association at Moderately High Concentration Via Composition-Gradient Static Light Scattering. J. Pharm. Sci. 105, 2310–2318 (2016).
- Esfandiary, R. et al. A systematic multitechnique approach for detection and characterization of reversible self-association during formulation development of therapeutic antibodies. J. Pharm. Sci. 102, 3089–3099 (2013).
Featured Products
720009187, December 2025