Rapid LC-MS Analysis of Glycoproteins With a 20 mm Wide Pore HILIC Column
Abstract
To demonstrate the use of a 20 mm ACQUITY™ Premier Glycoprotein BEH Amide, 300 Å, 1.7 µm Column for rapid glycoprotein separation and LC-MS analysis in HILIC mode.
Introduction
Due to the rapid growth of biopharmaceuticals such as monoclonal antibodies (mAb), high throughput methods are desirable in the entire development process. Hydrophilic Interaction Chromatography (HILIC) has been adopted as a useful tool for characterizing protein glycosylation, largely because of its ability to separate highly polar species. Wide-pore amide-bonded stationary phases have been shown to achieve remarkable separations of intact protein glycoforms.1,2 We show in this technology brief that employing a short, 2.1 x 20 mm ACQUITY Premier Glycoprotein BEH™ Amide, 300 Å, 1.7 µm Column (p/n: 186011017), can provide effective LCMS mass data for glycoproteins with more than 5 times faster analysis times compared to a 150 mm column.
Results and Discussion
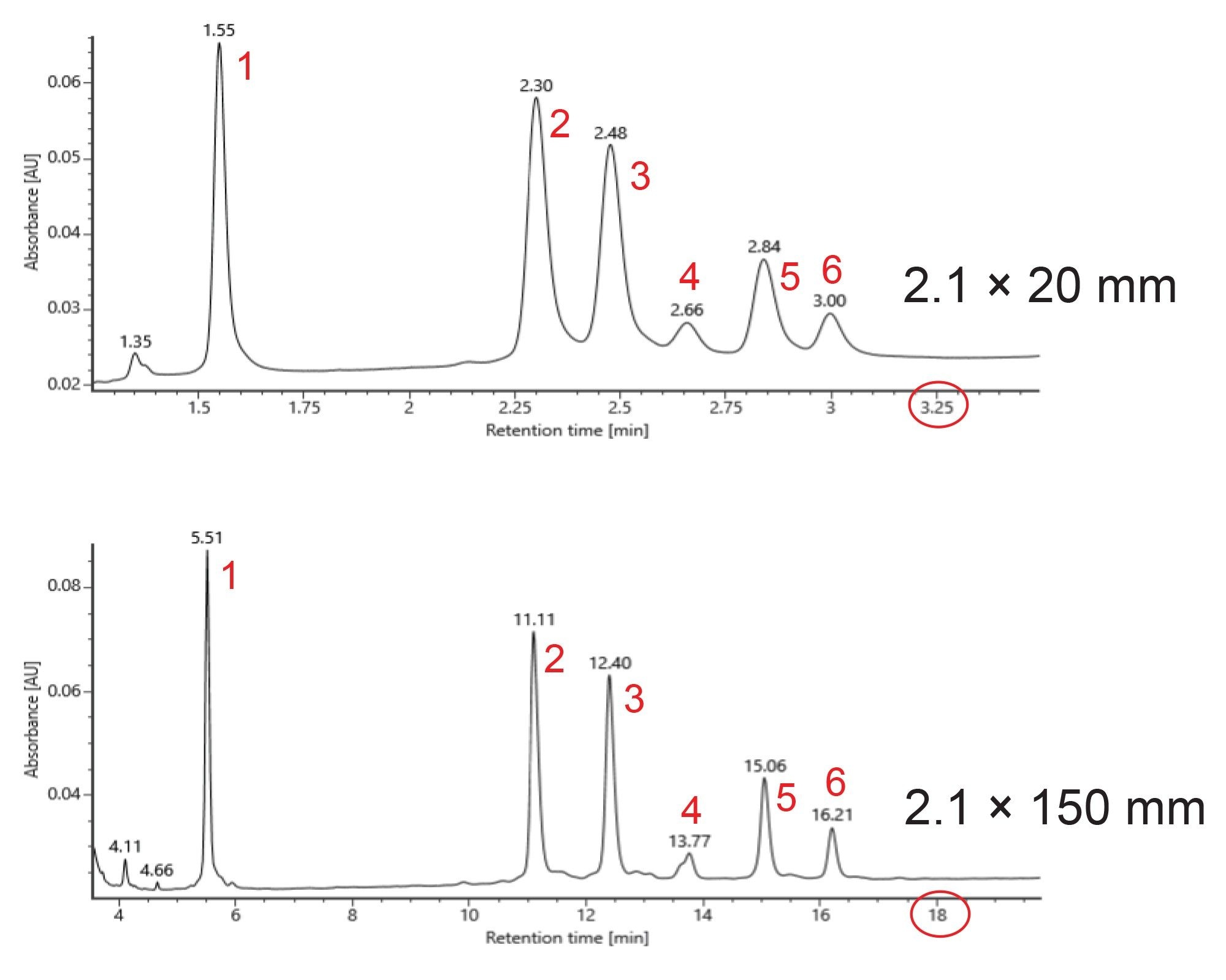
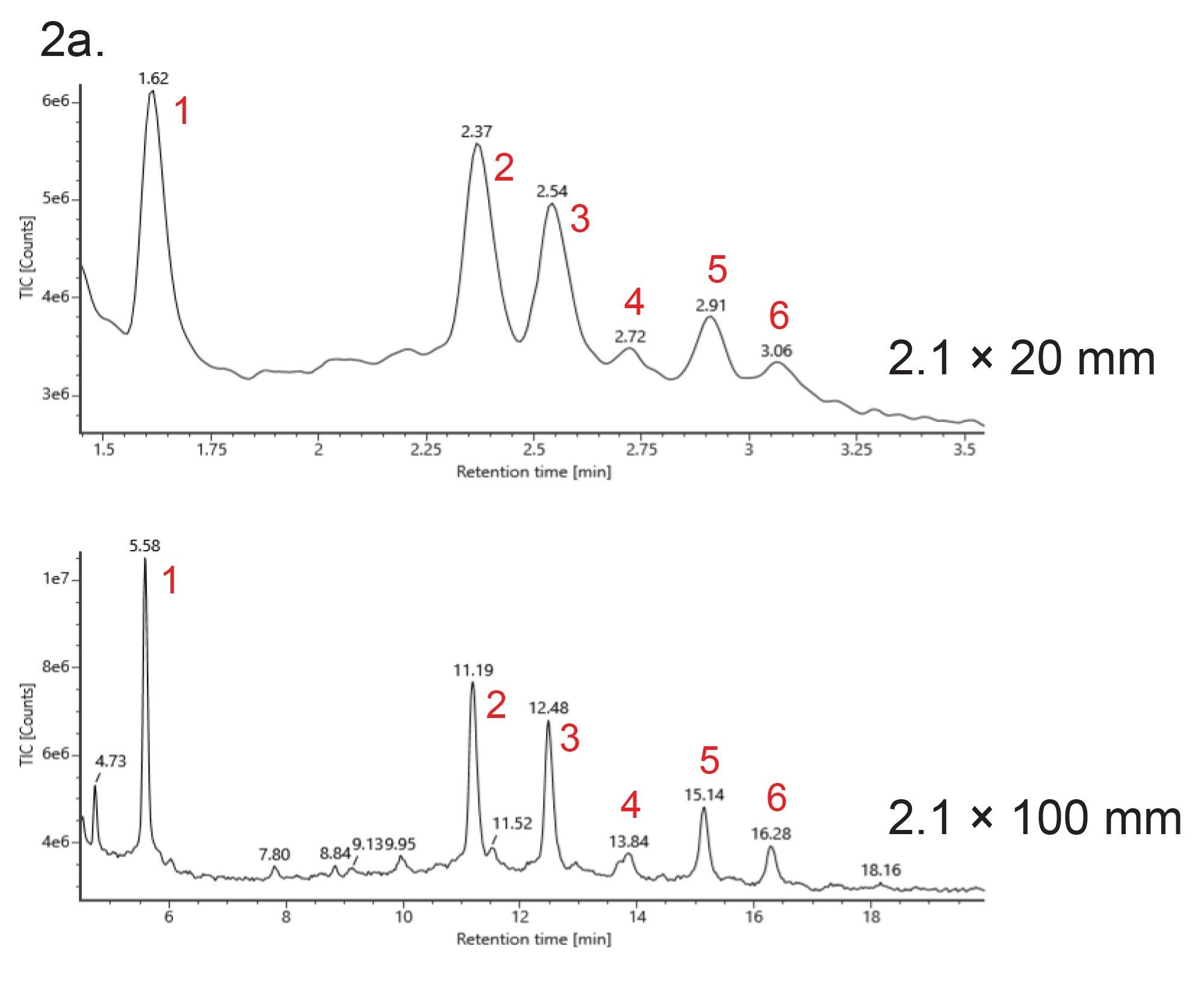
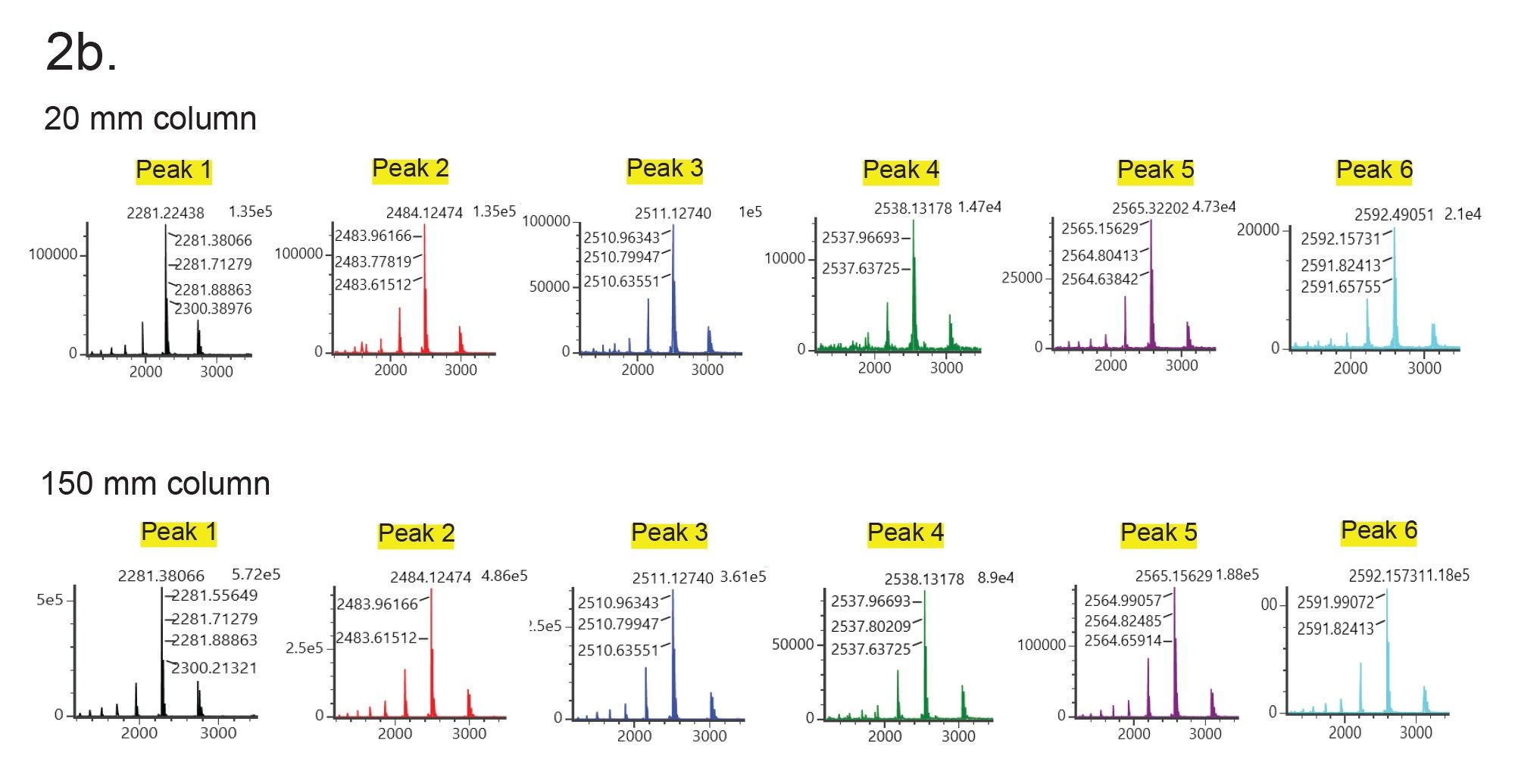
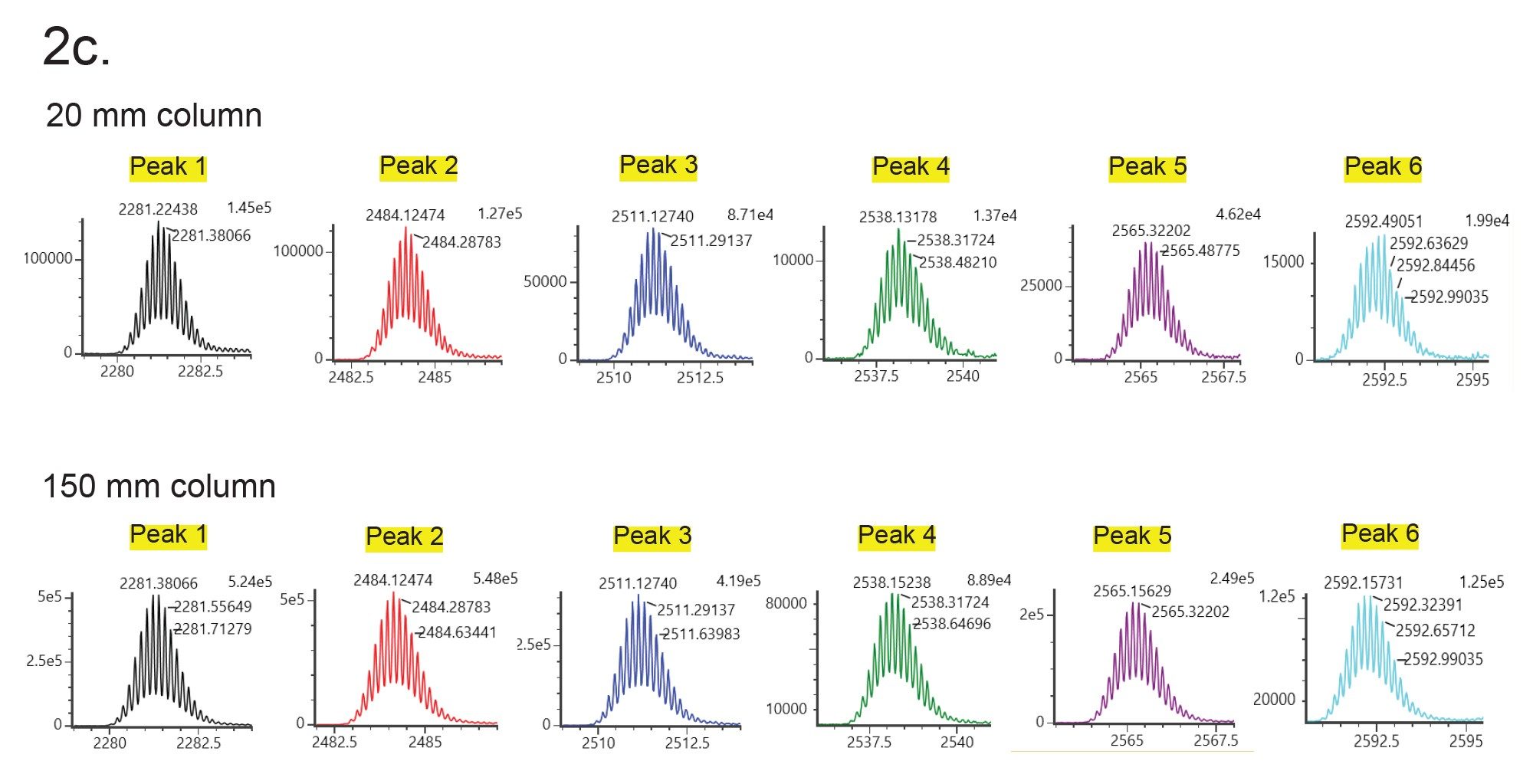
Figures 1 and 2a show UV and MS chromatograms of Glycoprotein Performance Test Standard (p/n: 186008010) separation using a 2.1 x 20 mm column and a 2.1 x 150 mm column with the same gradient slope with regard to column volumes, and an acetonitrile:water gradient (0.1% TFA). The test standard contains RNase A (aglycosylated form) and RNase B with its glycosylated forms (Man5 to Man9). Although loss of resolution is observed among major peaks, the MS data obtained on the 20 mm column and the 150 mm column were comparable (Figure 2b and 2c). The deconvoluted masses obtained from the 20 mm column and the 150 mm column were also consistent (Table 1). Importantly, the analysis time is more than 5 times faster using the 20 mm column than when using the 150 mm column. As an added benefit, mobile phase use is reduced over seven 7-fold and sample volume can be lowered 4-fold.
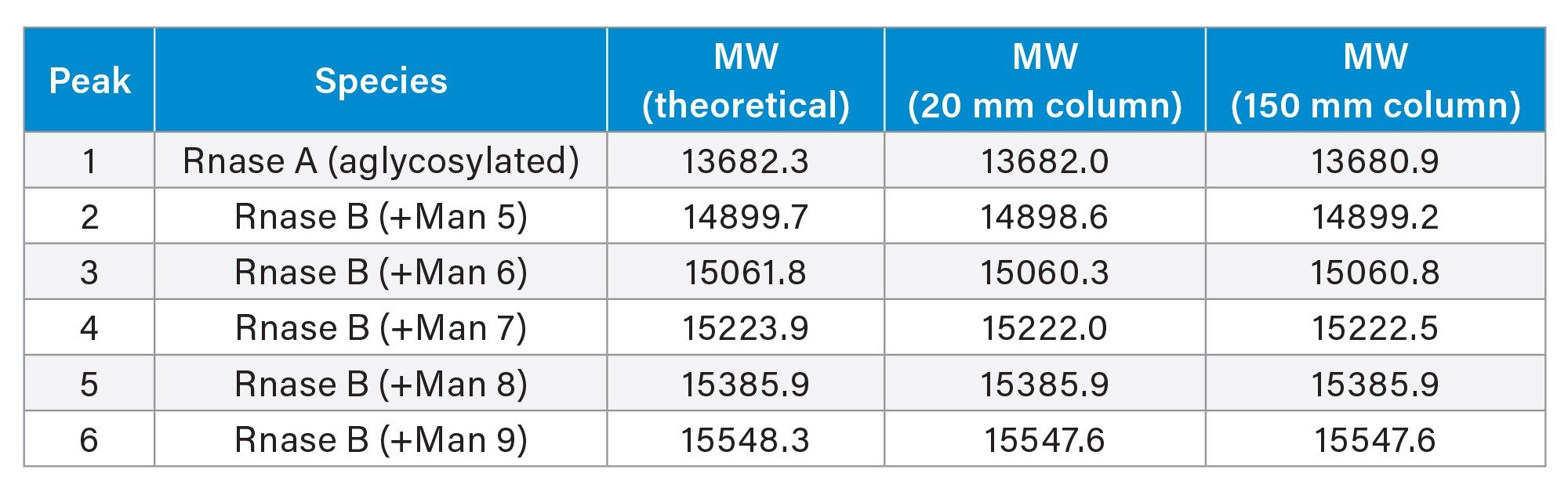
Conclusion
A 20 mm ACQUITY Premier Glycoprotein BEH Amide, 300 Å, 1.7 µm HILIC Column can effectively separate RNase A and RNase B glycoforms. The analysis time is more than 5 times faster using the 20 mm column than using the 150 mm column and consumes significantly lower quantities of acetonitrile. Although there is reduced resolution between the RNase glycoforms, the MS data were comparable between the 20 mm and 150 mm columns leading to high degree of confidence for glycoprotein identification in high-throughput methods.
References
- Lauber M.A., McCall S.A., Alden B.A., Iraneta P.C., and Koza S.M. Developing High Resolution HILIC Separations of Intact Glycosylated Proteins Using a Wide-Pore Amide-Bonded Stationary Phase. Waters Application Note. 720005380. April 2015.
- ACQUITY UPLC Glycoprotein BEH Amide, 300 Å, 1.7 µm Columns, ACQUITY Premier Glycoprotein BEH Amide, 300 Å, 1.7 µm Column, and Glycoprotein Performance Test Standard. Waters Care and Use Manual. 720005408. 2021.
720008304, March 2024