Analysis of Free Testosterone in Serum using the Xevo TQ Absolute for Clinical Research
Solo para fines de investigación. No se debe utilizar para procedimientos de diagnóstico.
Abstract
Described here is a clinical research method for the analysis of free testosterone in serum, using equilibrium dialysis and the Xevo™ TQ Absolute Mass Spectrometer to provide ultimate analytical sensitivity.
The clinical research method used a small amount of serum and a liquid-liquid extraction procedure following equilibrium dialysis. An ACQUITY™ UPLC™ I-Class PLUS FL System was used to separate testosterone on an ACQUITY BEH™ C18 Column, with detection using multiple reacting monitoring (MRM) on a Xevo TQ Absolute Mass Spectrometer. The developed method showed good analytical sensitivity and selectivity over a range of 1–500 pg/mL using 200 µL serum, with precision ≤8.4% CV across the range.
Benefits
- Excellent analytical sensitive analysis of free testosterone (0.5 pg/mL) from only 200 µL of serum
- Short equilibrium dialysis time of two hours
- Prepared sample volume allows for re-analysis if required
Introduction
The analysis of free testosterone for clinical research has been challenging in the past, mainly due to free testosterone making up <3% of the total testosterone, which presents analytical sensitivity challenges, and because equilibrium dialysis is a complex and time-consuming process.
However, the challenges were overcome by using the Xevo TQ Absolute and by using Rapid Equilibrium Dialysis (RED) Inserts and Plates from Thermo Scientific™ using a fast and controlled incubation. An analytically sensitive, precise, and accurate research method for free testosterone analysis has been developed.
An equilibrium dialysis procedure separated free testosterone from bound testosterone in serum and then used liquid-liquid extraction to concentrate and clean the free testosterone into a different solvent. The final extracts were injected on the ACQUITY UPLC I-Class PLUS FL System where free testosterone was separated on the ACQUITY BEH C18 Column and detected by the Xevo TQ Absolute Mass Spectrometer (Figure 1).
Experimental
Sample Description
A 1.0 mg/mL testosterone Certified Reference Material (CRM) (Merck, UK) solution was used to prepare a series of calibrators over the range 1–500 pg/mL in 52.75 mM HEPES buffer adjusted to pH 7.4 diluted from a 1 M stock (Merck, UK).
In-house Quality Control samples (QCs) were prepared using stripped MSG4000 serum (Golden West Biologicals, USA), unstripped serum and individual male serum samples (BioIVT, UK) at concentrations of approximately 2.79, 8.80, and 134 pg/mL.
A 100 µg/mL testosterone-13C3 Certified Reference Material (CRM) (Merck, UK) solution was used to prepare the working internal standard.
Equilibrium Dialysis
RED Device Inserts (Thermo Scientific, UK) were added to a RED Device Reusable Base Plate (Thermo Scientific, UK). 200 µL of serum was added to the matrix chamber and 400 µL of 52.75 mM HEPES buffer, adjusted to pH 7.40 ± 0.03, was placed in the buffer chamber. The plate was sealed with Sealing Tape (Thermo Scientific, UK) and placed into a temperature calibrated orbital shaker at 37 °C, mixing at 800 r.p.m. for two hours.
Liquid-Liquid Extraction
The RED Device Insert was removed, leaving the diasylate containing the free hormone in the well of the RED Device Reusable Base Plate. 300 µL of diasylate was transferred to a 2 mL microcentrifuge tube and 30 µL 1000 pg/mL testosterone-13C3 in methanol was added. Following a 30 second mixing step (2500 r.p.m. on a multi-tube vortex mixer), 1.5 mL of methyl tert-butyl ether was added, the tubes capped, shaken (five minutes at 2500 r.p.m.) and centrifuged at 16100 g for two minutes. 1.3 mL of supernatant was transferred to a clean Waters 2 mL 96 well sample collection plate (p/n: 186002482) and dried using nitrogen and a temperature of 40 °C. Finally, 70 µL of 50/50 v/v mobile phase A:mobileA: mobile phase B was added, the plate was sealed with a Waters square well cap mat (p/n: 186002484), shaken for 30 s at 2500 r.p.m. and then analyzed.
LC Conditions
|
Column: |
ACQUITY UPLC BEH C18 Column, 130 Å, 2.1 mm x 100 mm, 1.7 µm (p/n: 186002352) |
|
Column temperature: |
50 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
25 µL |
|
Injection mode: |
Partial Loop |
|
Needle size: |
20 µL |
|
Sample syringe: |
250 µL |
|
Sample loop: |
50 µL |
|
Flow rate: |
Refer to Gradient Table |
|
Mobile phase A: |
0.2 mM ammonium fluoride in water |
|
Mobile phase B: |
0.2 mM ammonium fluoride in methanol |
|
Weak wash: |
5% (v/v) methanol in water |
|
Strong wash: |
25/25/25/25 (v/v/v/v) acetonitrile/methanol/IPA/water |
|
Run time: |
4.5 minutes |
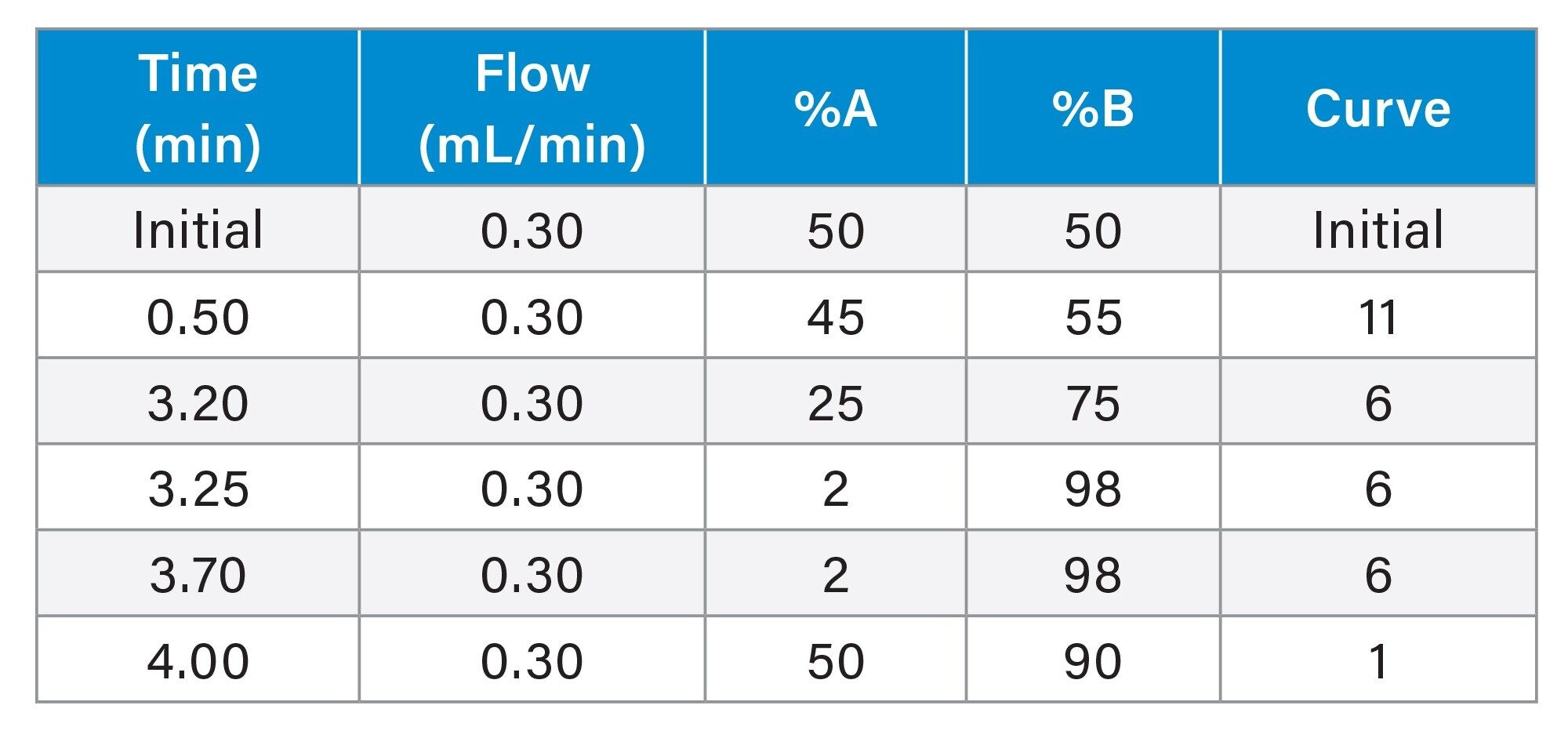
Gradient Table
MS Conditions
|
MS system: |
Xevo TQ Absolute Mass Spectrometer |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
1.2kV |
|
Desolvation temperature: |
650 °C |
|
Source temperature: |
150 °C |
|
Desolvation gas: |
1000 L/Hr |
|
Cone gas: |
150 L/Hr |
|
MS1 resolution: |
Unit (0.7 Da) |
|
MS2 resolution: |
Unit (0.7 Da) |
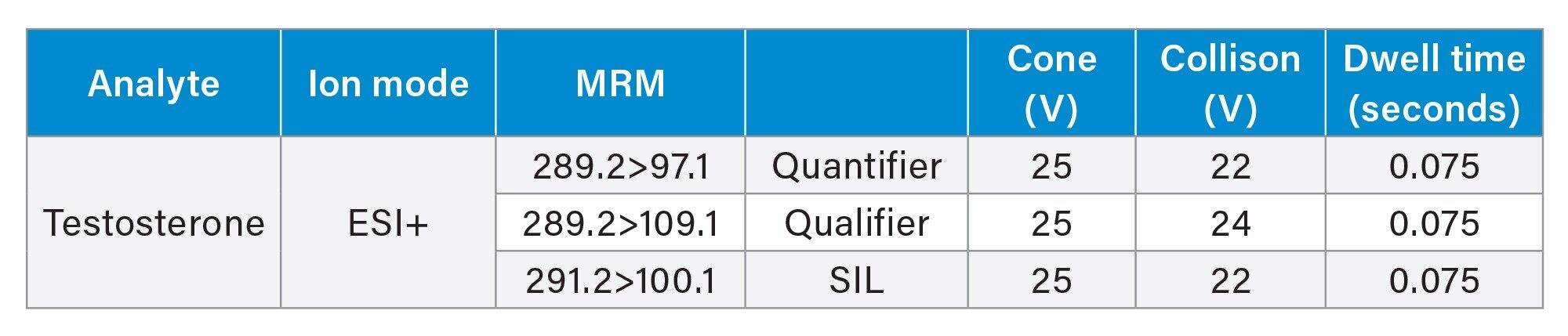
MRM Parameters
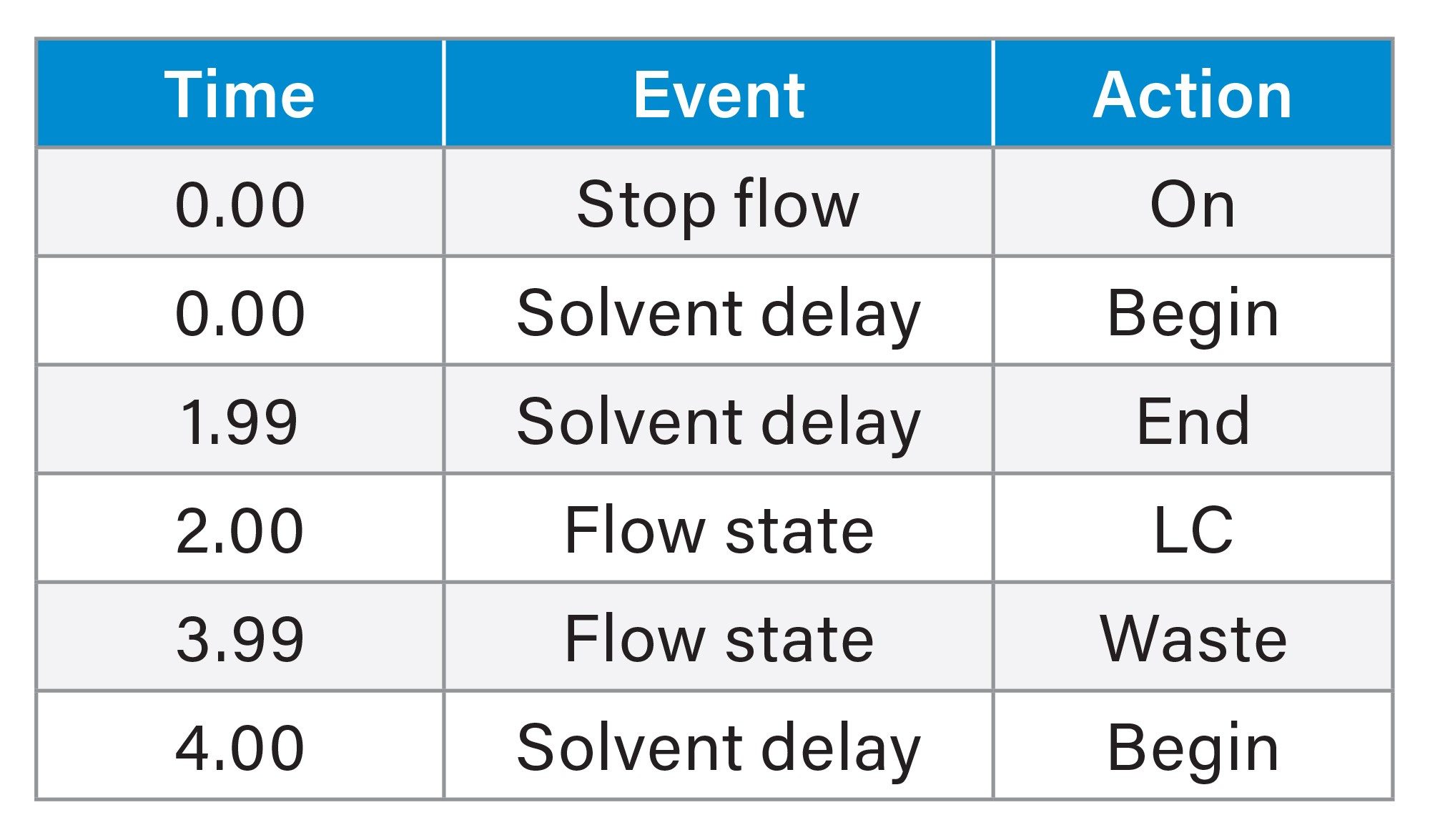
Method Events
Data Management
|
MS software: |
MassLynx™ v4.2 Software (SCN 1042) |
|
Informatics: |
TargetLynx™ XS v4.2 Application Manager |
Results and Discussion
The ACQUITY UPLC BEH C18 Column, 130 Å, 2.1 mm x 100 mm, 1.7 µm was used for the chromatographic retention of the free testosterone and its internal standard in a region of relatively low background noise. The injection-to-injection cycle time is 5.3 minutes, allowing for the analysis of 11 samples an hour. A 25 pg/mL testosterone solution in 50/50 v/v mobile phase A/mobile phase B was used for System Suitability Testing to benchmark the analytical sensitivity of the system and ascertain low chromatographic baseline noise before analysis.
The clinical research method was shown to be linear from 0.5–650 ng/mL, with r2 ≥ 0.995 across all analytical runs. No significant carryover into blank samples was observed from samples at 500 pg/mL. Final extracts were found to be stable for 24 hours at 10 °C in the Sample Manager.
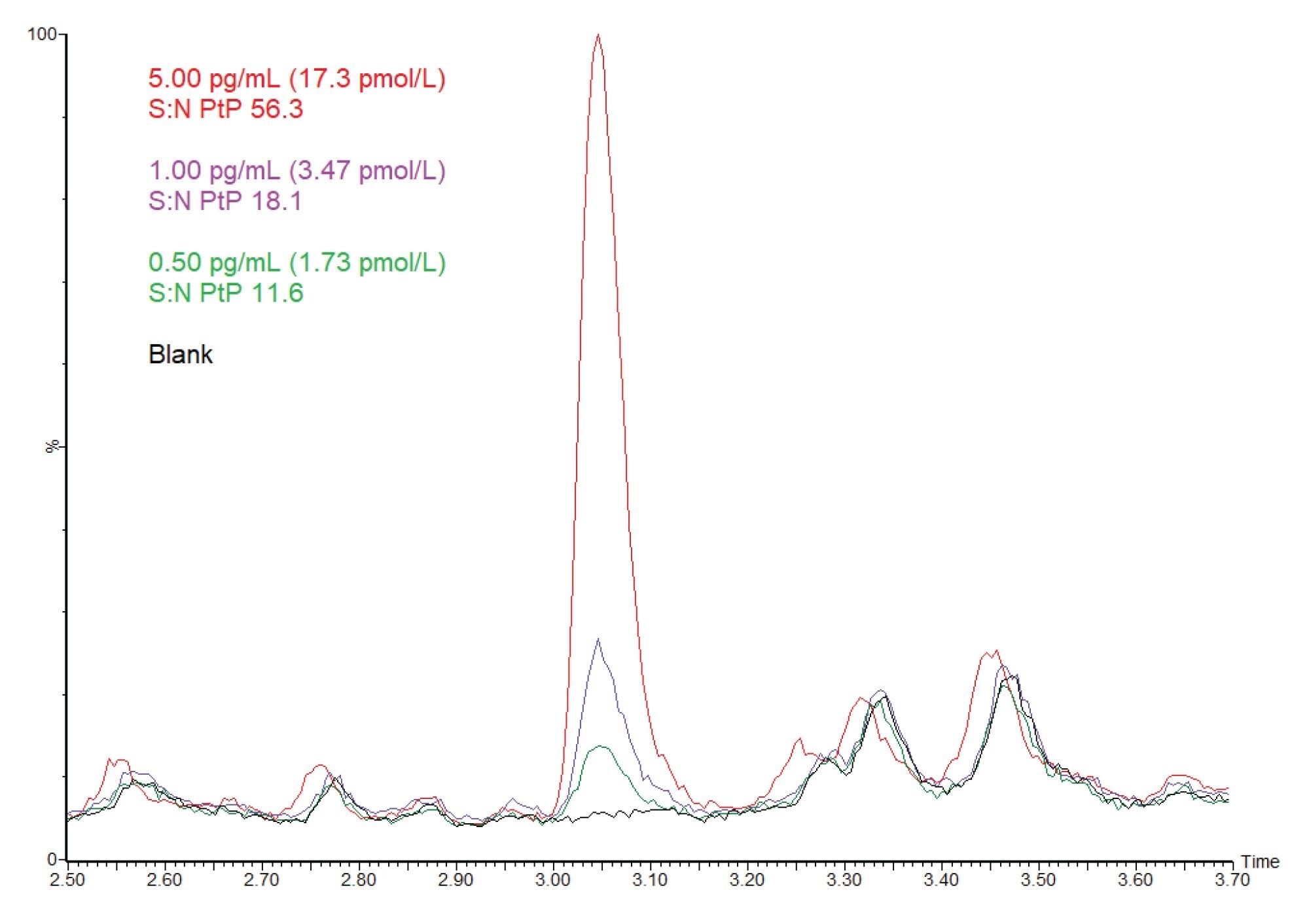
Analytical sensitivity investigations were performed across low concentrations of testosterone in 52.75 mM HEPES buffer adjusted to pH 7.4, at 0.20, 0.25, 0.30, 0.40, 0.50, 0.75, and 1.00 pg/mL, with ten replicates across five analytical runs (n=50). Investigations demonstrated a Lower Limit of the Measuring Interval (LLMI), the concentration at which precision is ≤20% and bias ≤15%, of 0.5 pg/mL was achievable. Results are presented in Table 1.

Representative chromatograms showing a blank 52.75 mM HEPES buffer extract, a 0.50 pg/mL extract (representing the LLMI), a 1.00 pg/mL extract (the calibrator 1 concentration), and a 5.00 pg/mL extract are shown in Figure 2, highlighting the analytical sensitivity achieved using this clinical research method.
Matrix effects were evaluated quantitatively by extracting MSG4000 stripped serum in triplicate and post-spiking samples with a low and high concentration of testosterone. Control samples were prepared in the same manner using 52.75 mM HEPES buffer adjusted to pH 7.4. Matrix effects based on peak area ranged from 96% to 102%, with ≤2.4% RSD, demonstrating neither ion enhancement nor suppression.
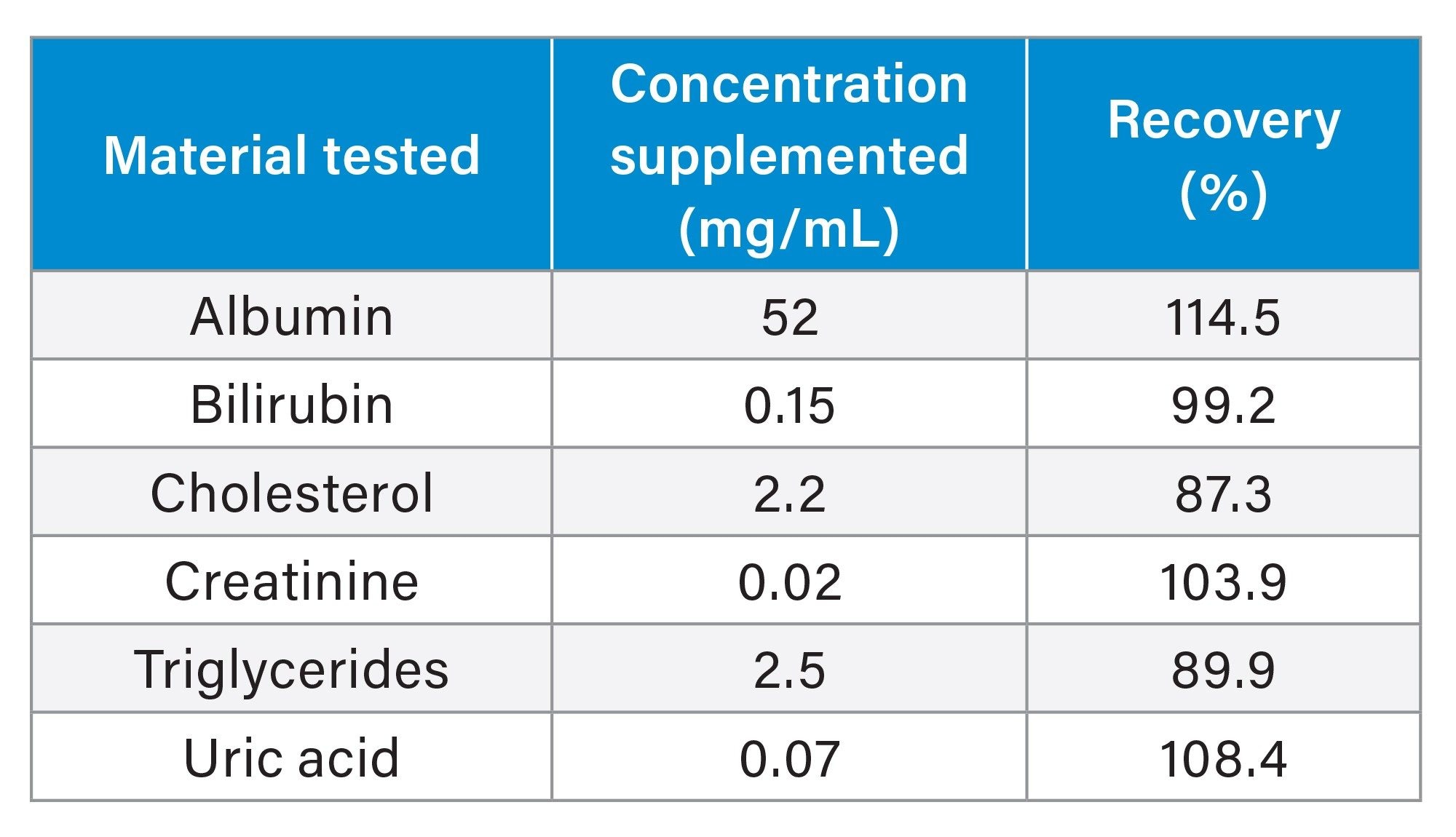
Interference testing was performed using a range of endogenous compounds (albumin, bilirubin, cholesterol, creatinine, triglycerides, and uric acid) at high concentrations. Test and control samples were analyzed in triplicate in a pooled serum sample with a free testosterone concentration of approximately 25 pg/mL. No significant interference was observed with recoveries ranging from 87.3–114.5. A summary is presented in Table 2.
Total precision and repeatability were evaluated for the method at low, medium, and high (2.79, 8.80, and 134 pg/mL) serum free testosterone QC concentrations over five analytical runs, with five replicates at each concentration (n=25). Total reproducibility and repeatability were ≤8.4% RSD. Figure 3 presents the results of these precision experiments.

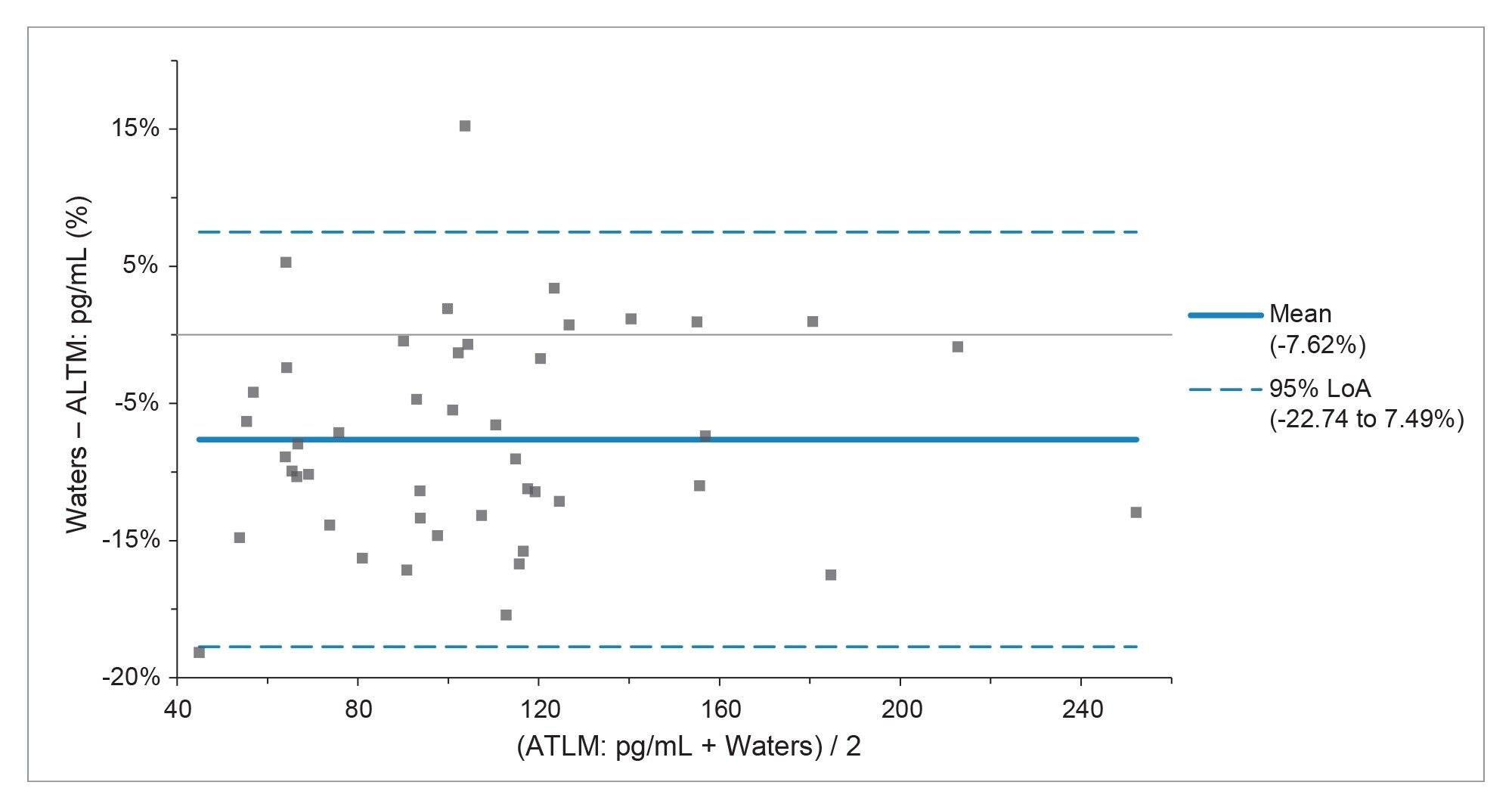
To evaluate method performance, 45 male serum samples ranging in concentration from 50.1–268.6 pg/mL from UK NEQAS were analyzed and the results were compared to the method All Laboratory Trimmed Mean (ALTM). Both a Passing-Bablok regression and Bland-Altman agreement were applied to the data set to assess the performance observed compared to the ALTM values. The Passing-Bablok regression showed an equation of y=-2.869 + 0.947x and the Bland-Altman agreement (Figure 4) demonstrated a small negative mean bias of -7.6% with a strong overall agreement.
Conclusion
We developed an LC-MS/MS clinical research method for measuring serum free testosterone using equilibrium dialysis, the ACQUITY UPLC I-Class PLUS FL System, and the Xevo TQ Absolute Mass Spectrometer. The method detected free testosterone concentrations as low as 0.5 pg/mL from 200 µL of serum and allows for sample for re-analysis if needed.
- The method demonstrates good linearity, with no significant carryover, interference, or matrix effects
- The method total reproducibility and repeatability was ≤8.4% RSD
- The method has strong agreement with the UK NEQAS external quality assurance scheme
Finally, the method is fast and efficient, as the equilibrium dialysis step takes only two hours and a full plate consisting of 48 samples can be prepared in less than five hours.
720008237, February 2024