The Arc Premier System (BSM-R) as a Flexible LC Platform for Peptide Mapping Assays
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
Peptide mapping assays are commonly deployed across biopharmaceutical labs to determine attributes associated with protein-based therapeutics such as protein sequence, post-translational modifications, drug product identity, and impurities. In this context, LC platforms that can deliver consistent results and be deployed across an organization are highly desirable. The Arc Premier System with MaxPeak High Performance Surfaces Technology is designed as a flexible LC platform that can be readily deployed in development and manufacturing of biopharmaceuticals. In this study, two condition sets, typical of development and manufacturing environments, were evaluated for peak retention time and tailing to assess system performance in terms of reproducibility and ability to mitigate adsorption of metal sensitive analytes. Results indicate the Arc Premier System featuring the binary solvent manager (BSM-R) is capable of delivering reproducible results across test conditions while minimizing analyte/surface interactions of metal-sensitive analytes. The results also conclude that it is suitable as a flexible LC platform that can be readily deployed in development and manufacturing environments.
Benefits
- The Arc Premier System configured with a binary solvent manager is capable of delivering reproducible results across a broad set of conditions
- MaxPeak High Performance Surfaces Technology improves chromatographic performance through reduced tailing of metal-sensitive peptides
Introduction
Peptide mapping is a method commonly deployed in the characterization and manufacturing of biopharmaceuticals. As an assay, peptide mapping is useful in determining protein sequence, post-translational modifications, drug product identity, and impurities associated with protein-based therapeutics. The complexity of the peptide map often requires demanding conditions such as lower flow rates and shallow gradients to deliver high resolving gradients for upstream activity particularly when characterizing drug candidates. However, given the ubiquitous nature of peptide mapping assays and their deployment across industry, it is not uncommon for peptide mapping assays to be migrated across labs and LC platforms. This can present challenges as instrument portfolios and performance can vary between labs which can impact assay results. Recently, Waters introduced the Arc Premier LC product line featuring MaxPeak High Performance Surfaces (HPS) Technology. The Arc Premier System is designed as a flexible LC platform which can be configured with a quaternary or binary solvent manager for easy deployment across labs to support development and manufacturing activities with improved performance towards metal-sensitive analytes. To demonstrate the Arc Premier System configured with the binary solvent manager as a suitable LC platform that can be deployed across an organization, a peptide mapping assay was evaluated under conditions typical of development and manufacturing environments. Peak retention time and tailing were used to assess system performance in terms of reproducibility and ability to mitigate adsorption of metal sensitive analytes.
Results and Discussion
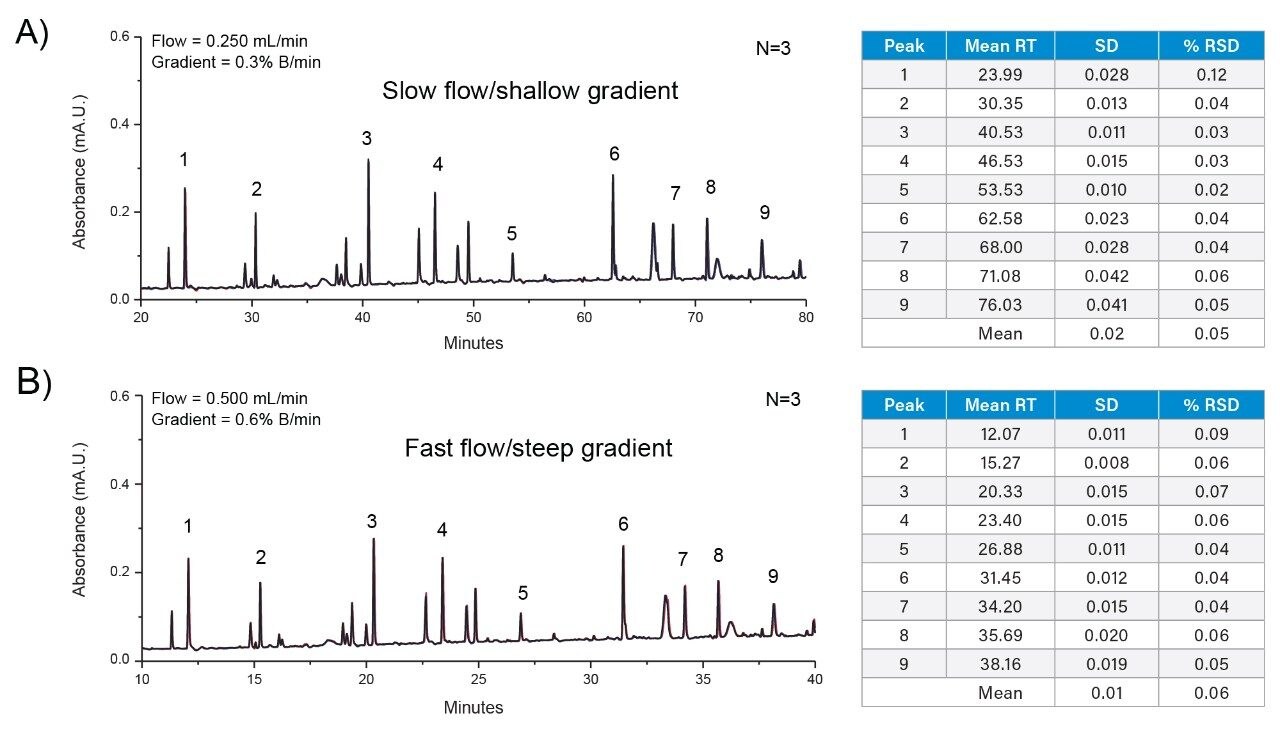
For this study, two methods were created representative of conditions that may be encountered in peptide mapping assays during the development and manufacturing of biopharmaceuticals. The first method represents a high-resolution separation typically deployed in upstream activities during the characterization and early process development phase of biotherapeutics. In this instance, the method uses a slower flow rate of 0.250 mL/min with a shallow gradient of 0.3% B/min which affords higher resolving power towards critical pairs but requires increased performance in the LCs ability to deliver the same gradient composition over repeated injections. The second method represents conditions that may be encountered in late-stage development or manufacturing environments where the bio process is well controlled and critical species are known and require monitoring. In this instance, a method that incorporates a faster flow of 0.500 mL/min and a steeper gradient (0.6% B/min) is used to accommodate the high throughput needs associated with these environments.
Using an XSelect Premier Column (2.5 µm, 4.6 × 150 mm, p/n: 186009874) recommended for the Arc Premier System, peptide mapping assays were performed as three replicate injections of Waters Tryptic Digestion Standard (p/n: 186009126) under both condition sets. As shown in Figure 1, peak profiles were highly comparable across condition sets and system reproducibility was evident based on the overlays of the replicate injections. To further probe the Arc Premier Systems ability to deliver consistent results, nine peaks were selected to compare retention time performance. As shown in the accompanying tables of Figure 1, mean retention time standard deviation (SD) was calculated to be 0.02 minutes (1.2 sec) and 0.01 minutes (0.5 sec) when using the slow flow/shallow gradient or fast flow/steep gradient, respectively with %RSD below 0.1% in both cases. This data demonstrates the Arc Premier System configured with a binary solvent manager is well suited for peptide mapping assays under both condition sets offering the flexibility to support development and manufacturing activities associated with biopharmaceuticals.
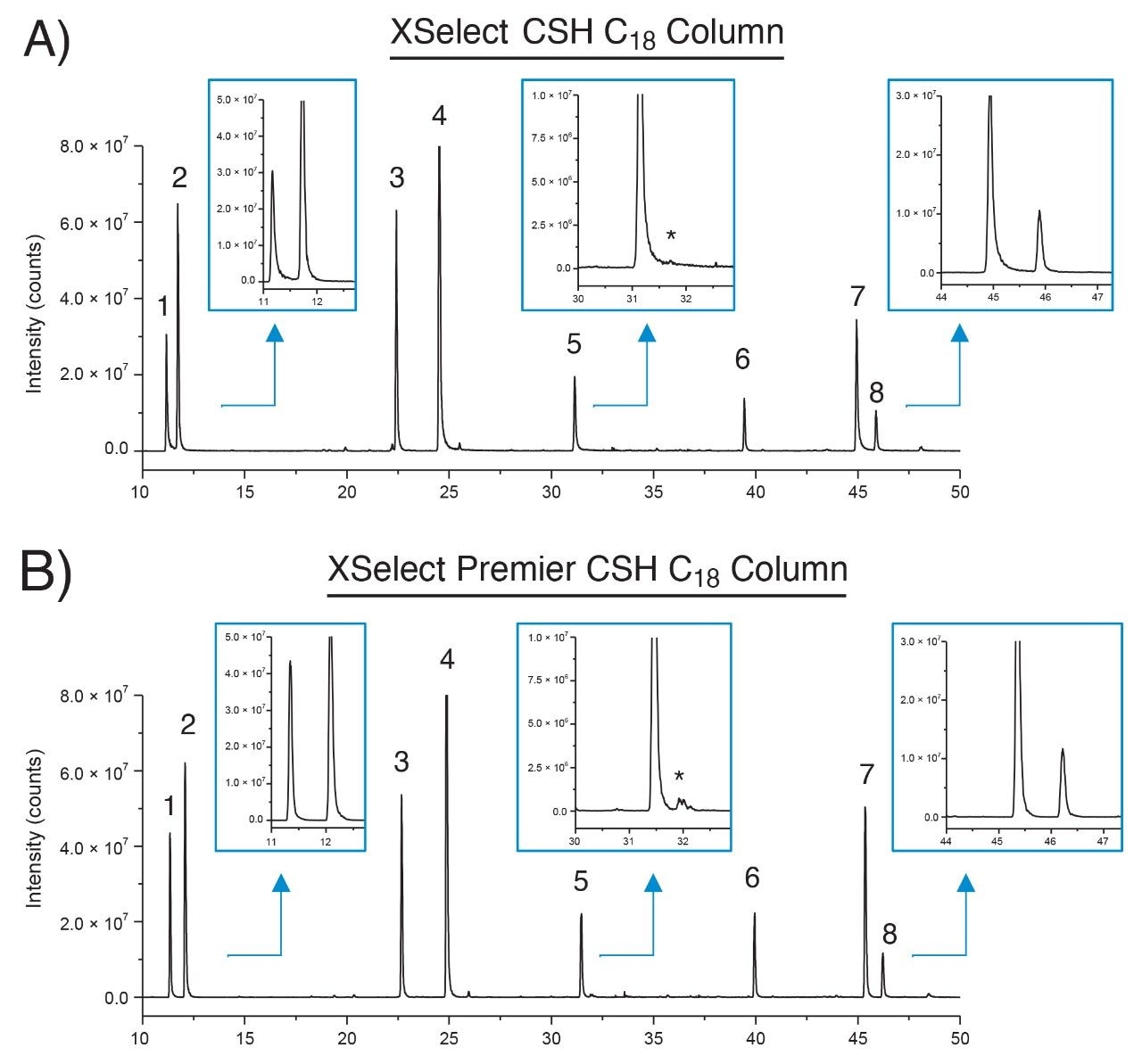
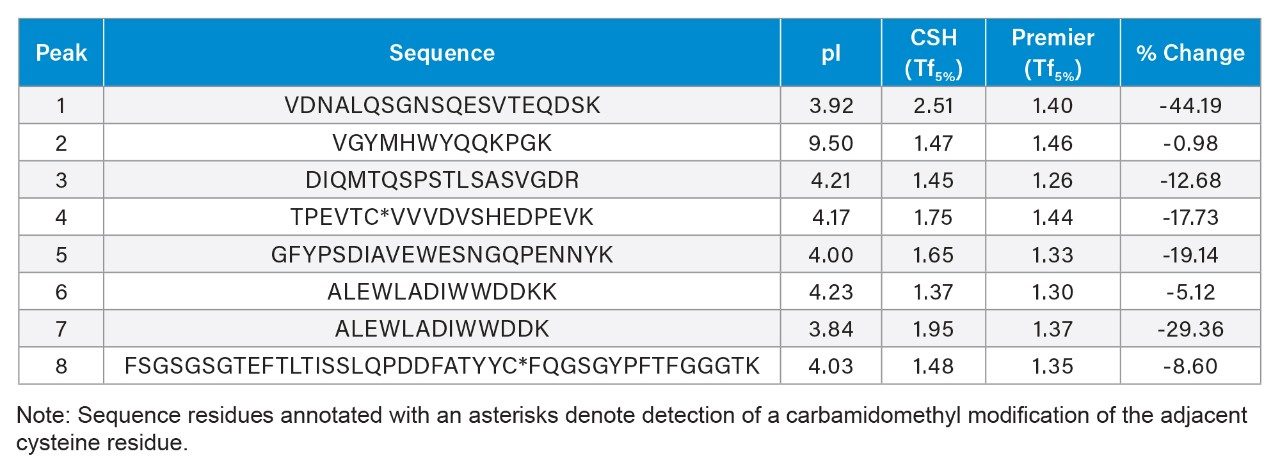
To investigate the performance of MaxPeak HPS Technology, the slow flow/shallow gradient conditions were used to perform a peptide mapping assay with an equivalent XSelect CSH Column (2.5 µm, 4.6 × 150 mm, p/n: 186006729) and compared to the previous data set acquired using the MaxPeak Premier branded column. Mass data was acquired for both separations using the ACQUITY QDa Mass Detector to facilitate comparison. Shown in Figure 2 are extracted ion chromatograms (XICs) for eight peptides that were previously observed to exhibit peak tailing differences when using MaxPeak HPS Technology.1 Upon visual inspection peak tailing was observed to be reduced most notably in peaks 1, 5, and 7 (insets) when using the MaxPeak Premier Column with MaxPeak HPS Technology. These observations were confirmed quantitatively where peaks 1, 5, and 7 were calculated to have the largest difference in peak tailing as shown in Table 1. While tailing was not as significant in the remaining peaks, a reduction in peak tailing was still observed. It should be noted that peak 2 was observed to have a marginal change in peak tailing which was not unexpected as peak 2 is considered to be a “basic” peptide (pI > 7.0) where the MaxPeak HPS Technology would not be expected to impact performance in terms of peak tailing. These results demonstrate MaxPeak HPS Technology is able to improve chromatographic performance by minimizing analyte/surface interactions of metal-sensitive analytes.
Conclusion
This study demonstrates the Arc Premier System (BSM-R variant) with MaxPeak HPS Technology offers exceptional chromatographic performance and is capable of delivering reproducible results across a variety of conditions while minimizing analyte/surface interactions of metal-sensitive analytes. The Arc Premier System is ideal as a flexible LC platform that can be readily deployed across labs to support development and manufacturing activities of biopharmaceuticals.
References
- Birdsall et al. Reducing Metal-Ion Mediated Adsorption of Acidic Peptides in RPLC-Based Assays Using Hybrid Silica Chromatographic Surfaces. Journal Chromatography. B. Vol 1179, 2021.
Featured Products
720007464, January 2022