For research use only. Not for use in diagnostic procedures.
This application note describes an analytically sensitive and selective clinical research method developed for the analysis of serum testosterone and androstenedione utilizing Oasis MAX μElution Plate Technology for the extraction of testosterone and androstenedione from serum, which has been automated on the Tecan Freedom Evo 100/4 Liquid Handler.
Testosterone and its precursor, androstenedione, are androgenic steroid hormones that are involved in the development and maintenance of sexual characteristics. Analysis of these structurally similar steroid hormones using UPLC-MS/MS provides three levels of selectivity: 1) sample preparation, 2) liquid chromatography, and 3) mass spectrometric detection using multiple reaction monitoring (MRM). LC-MS/MS, while analytically sensitive and selective, has been reported to suffer from a lack of agreement between laboratories when methods are independently developed using different calibration materials and employing manual extraction techniques that may introduce operator variability. The availability of a voluntary Hormone Standardization (HoSt) Program, co-ordinated by the United States Centers for Disease Control and Prevention (CDC), has begun to help address these harmonization issues.
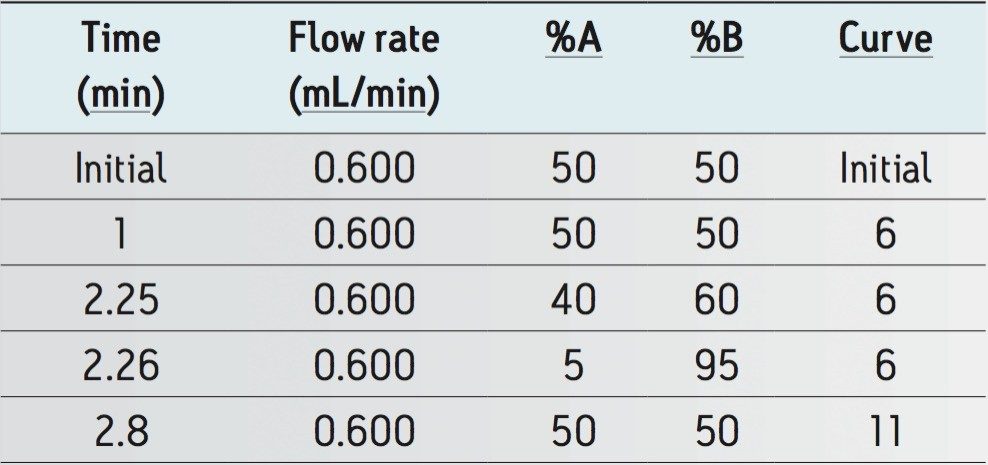
Here we describe a clinical research method utilizing Oasis MAX μElution Plate Technology for the extraction of testosterone and androstenedione from serum, which has been automated on the Tecan Freedom Evo 100/4 Liquid Handler. Chromatographic separation was performed on an ACQUITY UPLC I-Class System using an ACQUITY UPLC HSS SB C18 Column followed by detection on a Xevo TQD Tandem Quadrupole Mass Spectrometer (Figure 1), utilizing MassLynx Software v4.1 with TargetLynx Application Manager. In addition, the method has employed CDC HoSt testosterone samples to evaluate the accuracy and therefore suitability of the method for analysing testosterone for clinical research.

|
System: |
ACQUITY UPLC I-Class |
|
Needle: |
30 μL |
|
Column: |
ACQUITY UPLC HSS SB C18, 2.1 x 50 mm, 1.8 μm (p/n 186004118) |
|
Mobile phase A: |
water with 2mM NH4Ac + 0.1% formic acid |
|
Mobile phase B: |
methanol with 2mM NH4Ac + 0.1% formic acid |
|
Needle wash solvent: |
80% methanol + 0.1% formic acid |
|
Purge solvent: |
50% methanol + 0.1% formic acid |
|
Column temp.: |
60°C |
|
Injection vol.: |
20 μL |
|
Flow rate: |
0.60 mL/min |
|
Gradient: |
See Table 1 |
|
Run time: |
3.3 minutes |
|
System: |
Xevo TQD |
|
Resolution: |
MS1 and MS2 (0.7 FWHM) |
|
Acquistion mode: |
Multiple Reaction Monitoring (MRM) (see Table 2 for details) |
|
Polarity: |
ESI positive |
|
Capillary: |
0.50 kV |
|
Source Temp: |
140 °C |
|
Desolvation Temp.: |
450 °C |
|
Dwell Time: |
0.05 seconds |
|
Inter-scan delay: |
0.01 seconds |
|
Inter-channel delay: |
0.02 seconds |
MassLynx Software v4.1 with TargetLynx Application Manager
Testosterone, androstenedione, and their 13C3 labeled internal standards were purchased from Cerilliant (Round Rock, TX). MSG4000 stripped human serum was purchased from Golden West Biologicals (Temecula, CA). Using these materials, calibrators were prepared over the range of 0.17 – 52 nmol/L (0.05 – 15 ng/mL), with quality controls (QCs) at 0.52 nmol/L, 3.5 nmol/L, and 35 nmol/L (0.15, 1, and 10 ng/mL) for both testosterone and androstenedione.*
* To convert SI units (nmol/L) to conventional mass units (ng/mL) divide by 3.470 for testosterone and 3.494 for androstenedione.
Extraction was performed using a Tecan Freedom Evo 100/4 Liquid Handler. To 200 μL of sample; 25 μL of internal standard, 40 μL of 2% ammonia (aq), 150 μL 0.2M zinc sulphate, and 250 μL of methanol were added. 300 μL of water was added prior to centrifugation for 5 minutes at 1000 g. The samples were mixed after the addition of each reagent.
The Oasis MAX μElution SPE Plate (p/n 186001829) was conditioned and equilibrated with 200 μL methanol and water, respectively. An aliquot of each of the pretreated samples (600 μL) was loaded into individual wells of the plate and slowly pulled through at low vacuum. The plate was washed with 200 μL of 0.1% ammonia in 20% methanol and dried. The analytes were eluted using 2 x 25 μL methanol, followed by 50 μL water.
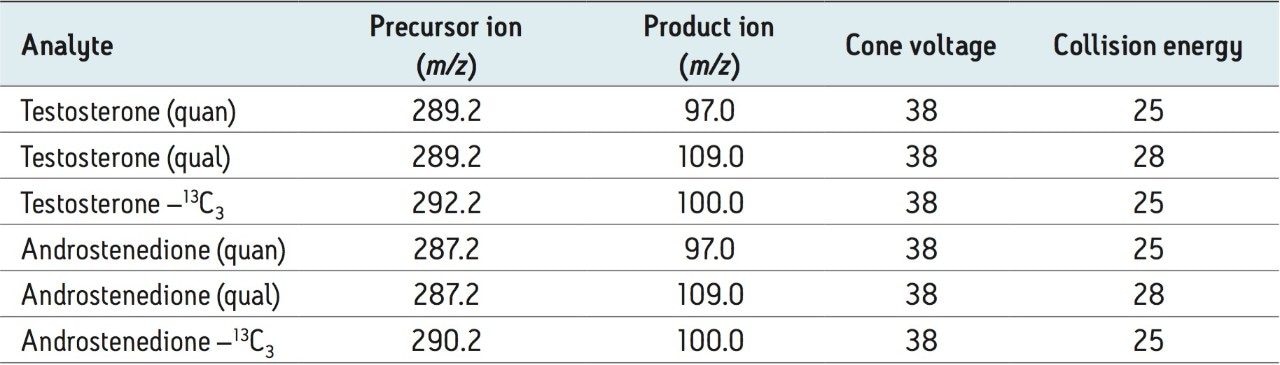
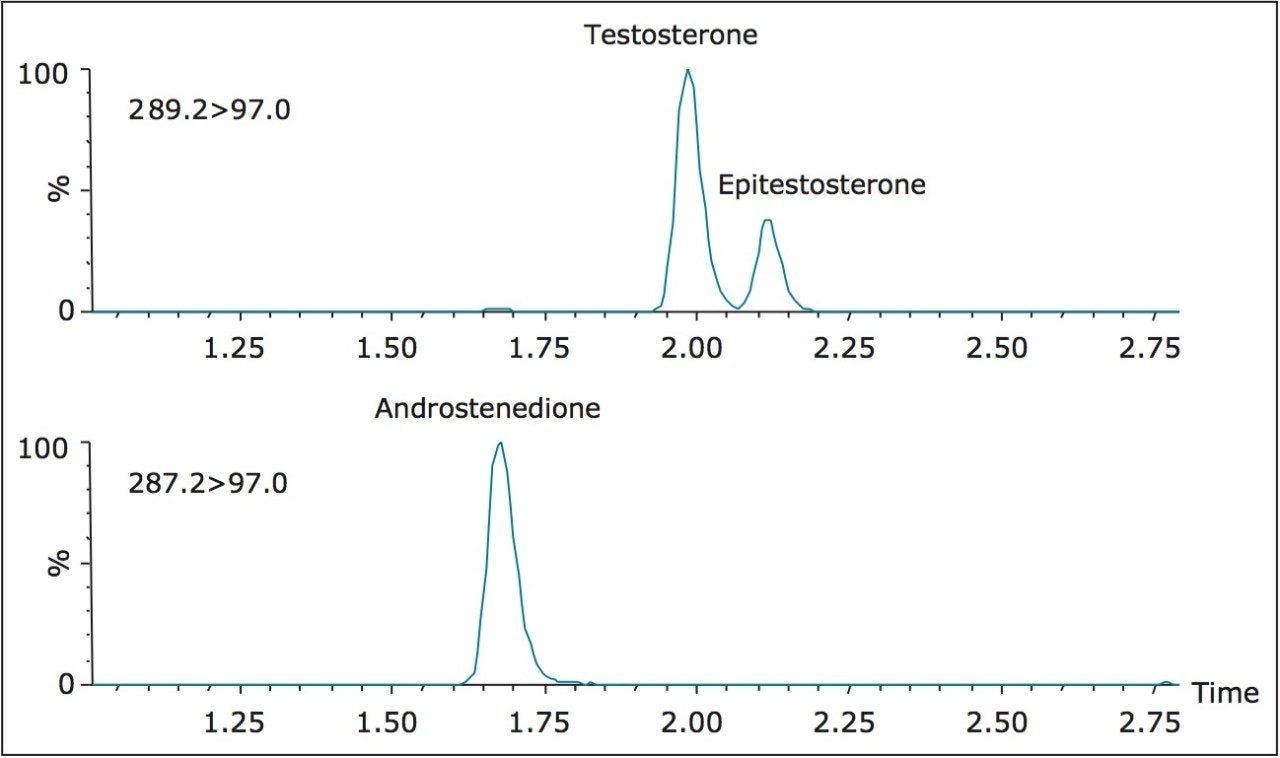
No interferences were observed at the retention time of both testosterone and androstenedione when eight structurally related compounds were examined (17-hydroxyprogesterone, epitestosterone, dihydrotestosterone, DHEA, DHEAS, 11-deoxycorticosterone, corticosterone, and 21-deoxycortisol). The chromatographic selectivity of the column is demonstrated through the baseline resolution of testosterone and its epimer; epitestosterone (Figure 2). Separation of androstenedione, 17-hydroxyprogesterone, and DHEA from testosterone is necessary because these analytes or their isotopes produce signals in the testosterone MRM trace at concentrations of 1 μmol/L.
No system carryover was observed in blank injections following measurement of high concentration samples (52 nmol/L) for both analytes. A 1:4 dilution was successfully performed on an over-range sample (102 nmol/L) with a mean accuracy of 98% (2.0% RSD) and 100% (3.1% RSD), for testosterone and androstenedione, respectively.
The method was shown to be linear over the range of 0.17 - 52 nmol/L when different ratios of high and low concentration pools of testosterone and androstenedione were combined and analysed. Calibration lines in spiked serum were linear with coefficient of determinations (r2) >0.994 over 10 separate occasions.
Analytical sensitivity investigations revealed that this method would allow precise quantification (<20% RSD) at 0.085 nmol/L for both testosterone and androstenedione. The lowest calibrator was established at 0.17 nmol/L. At this concentration the signal:noise (S/N) was consistently greater than 10:1, while maintaining <20% precision performance for both analytes.
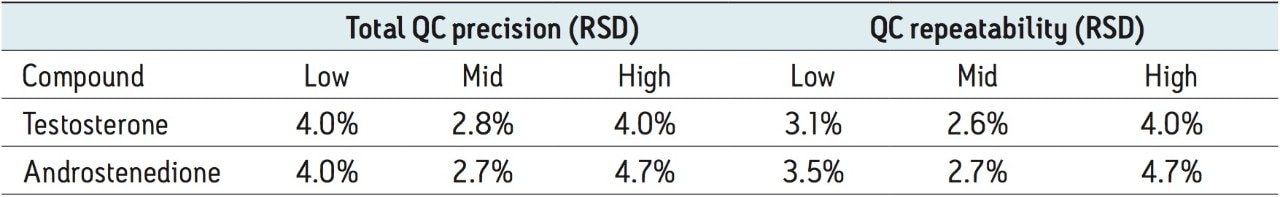
Total precision was determined by extracting and quantifying three replicates of tri-level QC material on two occasions per day over five separate days (n=30). Repeatability was assessed by analyzing three replicates at each QC level. The results of these experiments are seen in Table 3, where total precision and repeatability at the low (0.52 nmol/L), mid (3.5 nmol/L), and high (35 nmol/L) concentrations is ≤4.0% RSD for testosterone and ≤4.7% RSD for androstenedione.
Matrix effects were evaluated as the peak area of endogenous testosterone and androstenedione samples taken as a percentage of extraction solvent samples spiked to equivalent concentrations. Mean (range) matrix effects were 0.67 (0.55–0.78) for testosterone and 0.77 (0.69–0.86) for androstenedione. Calculations using analyte:internal standard response ratio indicated compensation for signal suppression by the internal standard, providing a mean (range) net matrix effect of 0.99 (0.88–1.18) for testosterone and 1.03 (0.89–1.22) for androstenedione.
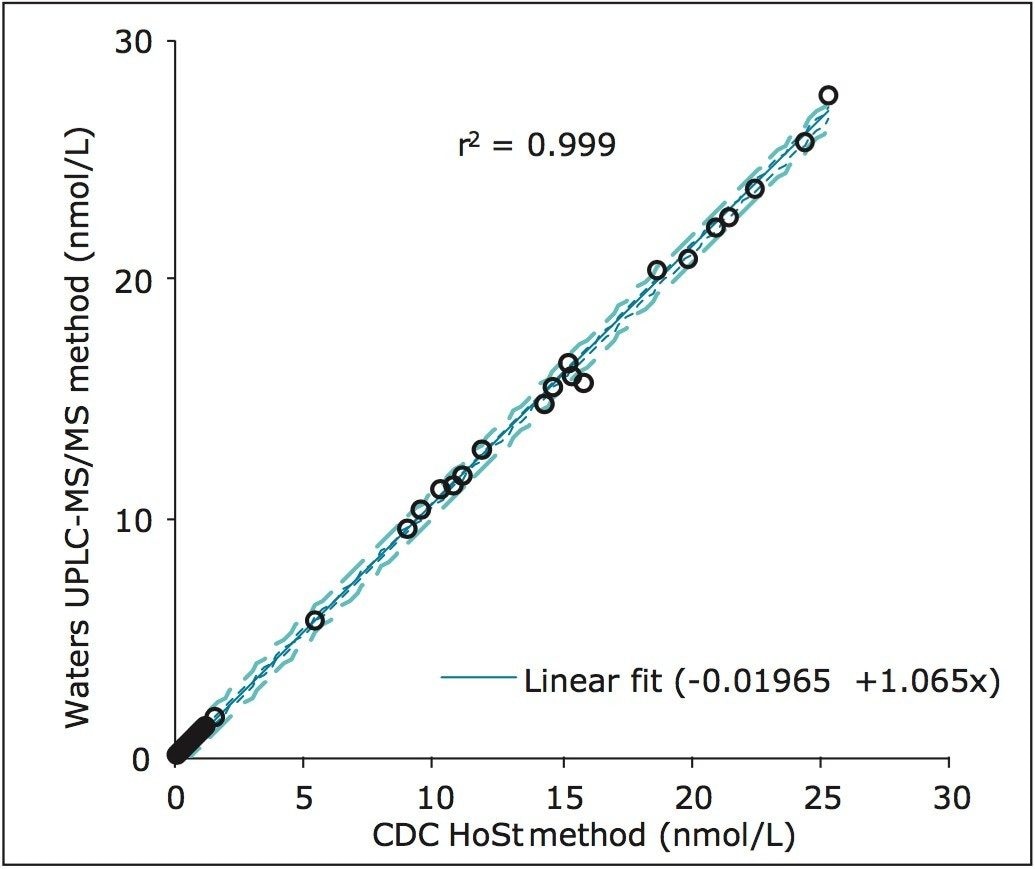
Phase I Hormone Standardization (HoSt) samples (CDC, Atlanta, GA) were used to evaluate the method accuracy for testosterone. Samples (n=40) were analysed in duplicate on two occasions over five separate days. Excellent correlation between the calculated values and assigned values for the CDC HoSt samples was demonstrated with a coefficient of determination (r2) of 0.999 (Figure 3). Phase 1 evaluation from the CDC demonstrated a 3.3% mean bias for this method which is within their ±6.4% bias acceptance limit.
A set of anonymized serum samples were selected (n=35) for comparison against an independently developed LC-MS/MS method for testosterone and androstenedione. Comparison data were processed using Analyse-it Software v2.3. The comparison between the two independent LC-MS/MS methods yielded a Deming regression of y = 1.07x + 0.04 for testosterone (Figure 4A), showing statistically significant proportional bias but no significant constant bias, and y = 0.96x + 0.08 for androstenedione (Figure 4B), showing no significant proportional or constant bias.

Using the same samples, a comparison was performed between this automated method and the same method but using manual SPE sample preparation for the analysis of testosterone and androstenedione. Comparison of the sample set yielded a Deming regression of y = 1.01x + 0.01 for testosterone, showing no significant proportional bias or constant bias, and y = 0.97x + 0.17 for androstenedione, showing no significant proportional or constant bias. This indicates equivalency of the manual and automated extraction methods, which allows for flexibility in sample preparation options for the LC-MS/MS analysis of both analytes.
An analytically sensitive and selective clinical research method has been developed for the analysis of serum testosterone and androstenedione.
Excellent method precision was achieved through the use of analytically sensitive and selective sample preparation, chromatography, and MRM mass spectrometry on the ACQUITY UPLC I-Class UPLC System and Xevo TQD. In addition, method accuracy for the analysis of testosterone has been demonstrated through evaluation of CDC HoSt samples.
The sample preparation has been automated using the Tecan Freedom Evo 100/4 Liquid Handler, significantly reducing sample handling time and improving laboratory efficiency with sample tracking capabilities. Both a manual and the automated extraction methods have been shown to be equivalent, providing the user with flexibility in sample preparation options.
Professor Brian Keevil and his colleagues at the Department of Clinical Biochemistry, University Hospital of South Manchester, Wythenshawe, UK, are thanked for the provision of anonymized plasma samples for this analysis.
720005274,January 2015