The Waters Neutrals QC Reference Material (QCRM) is a mixture of three neutral compounds that can be separated with common mobile phases with sufficient organic composition, and is compatible with most column chemistries, making it an ideal standard for troubleshooting HPLC and UPLC system problems. In this application note, six common chromatographic problems are examined to demonstrate the utility of the Neutrals QCRM in rapidly diagnosing problems on an HPLC or UPLC system.
Liquid chromatography is a powerful analytical method of analysis, however, when an HPLC or UPLC system begins to malfunction, it can mean a considerable amount of time and resources to fix. Some system problems, such as a leak in the pump, can be noticed by an experienced chromatographer, while other problems, such as improperly connected column outlet tubing, can be a subtle problem and difficult to troubleshoot. By using a qualified system suitability standard, a chromatographer can more easily detect problems within their system, potentially reducing system downtime.
Waters Neutrals Quality Control Reference Material (QCRM) is a mix of three neutral compounds: acetone, naphthalene, and acenaphthene. These compounds are manufactured with batch-to-batch reproducibility in a controlled setting ensuring consistent results over time. This standard is an ideal solution for system troubleshooting and maintenance as the separation of these compounds can be achieved under common mobile phase conditions with sufficient organic content. In this application, six common chromatographic problems are examined to demonstrate the utility of the Neutrals QCRM in rapidly diagnosing problems on an HPLC or UPLC system. After repairs were made, the Neutrals QCRM was used to quickly confirm that the system was back to operating optimally. By using the Neutrals QCRM to check system functionality, data quality can be assured and the system can be used with confidence.
|
Mobile phase: |
50:50 Acetonitrile:water |
|
Separation mode: |
Isocratic |
|
Detection (PDA): |
UV 254 nm |
|
Column: |
ACQUITY UPLC BEH C18 , 2.1 x 50 mm, 1.7 μm (p/n 186002350) |
|
Column temp.: |
30 °C |
|
Needle wash: |
50:50 ACN:water |
|
Sample purge: |
50:50 ACN:water |
|
Seal wash: |
50:50 MeOH:water |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
1 μL |
|
Data management: |
Empower 3 CDS |
A vial of ASR Neutral QC Reference Material (p/n 186006360) was opened and transferred into an LCGC Certified Clear Qsert Vial (p/n 186001126C) for injection
When problems arise on an HPLC or UPLC system, system troubleshooting can be a time consuming and costly process. By using a QC Reference Material (QCRM), a chromatographer can more easily identify problems with a system and make repairs, potentially reducing system downtime. Waters Neutrals QCRM is a mixture of acetone, naphthalene, and acenaphthene that is manufactured in a tightly controlled setting with batch-to-batch reproducibility, to ensure reliable results. The standard can be used to both benchmark system performance and troubleshoot system problems. Furthermore, the standard can be used after repairs to ensure the system is operating optimally.
It is beneficial to have a system’s performance benchmarked, in order to monitor system performance and ensure quality data generation.1 Subsequently, if any problems arise within the system, the operator can compare the performance after any repairs, to performance before the problem was present. In this application, six common chromatographic issues are examined, using the Neutrals QCRM to rapidly diagnose the problem, shown in Table 1. During this experiment retention time, USP tailing factor, and USP plate count were monitored. Although many other parameters may be monitored, these parameters were chosen since many methods, especially compendial methods, have requirements for these parameters. Furthermore, all of these parameters can be indicators of a malfunctioning system.
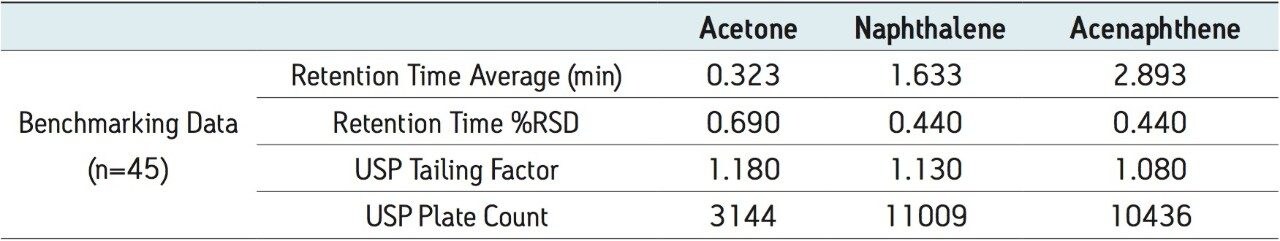
Forty-five injections of the Neutrals QCRM were run on a recently calibrated ACQUITY UPLC H-Class with ACQUITY UPLC PDA detector for five days, as shown in Table 2, prior to any system malfunction or user error. As part of a system benchmarking process, the operator should create appropriate specifications according to laboratory protocols that the Neutrals QCRM must pass in order for the system to be considered working optimally.2 By creating these specifications, the system performance can be monitored, potentially catching problems before they arise.
To showcase the troubleshooting capability of the Neutrals QCRM, the first issue demonstrated is the effect of a failing column on the separation. Over time, with repeated injections, all LC columns will lose their efficiency and ability to separate components of a mixture. In Figure 1, the separation of the Neutrals QCRM on an ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 µm Column that had been excessively used is shown and compared to the separation obtained on a column with acceptable performance.
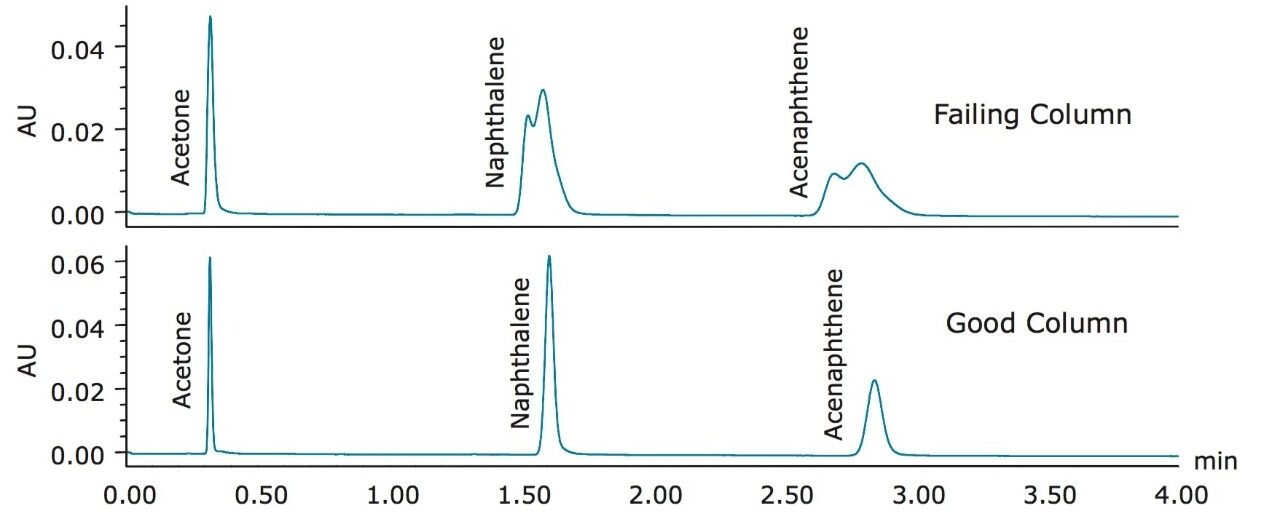
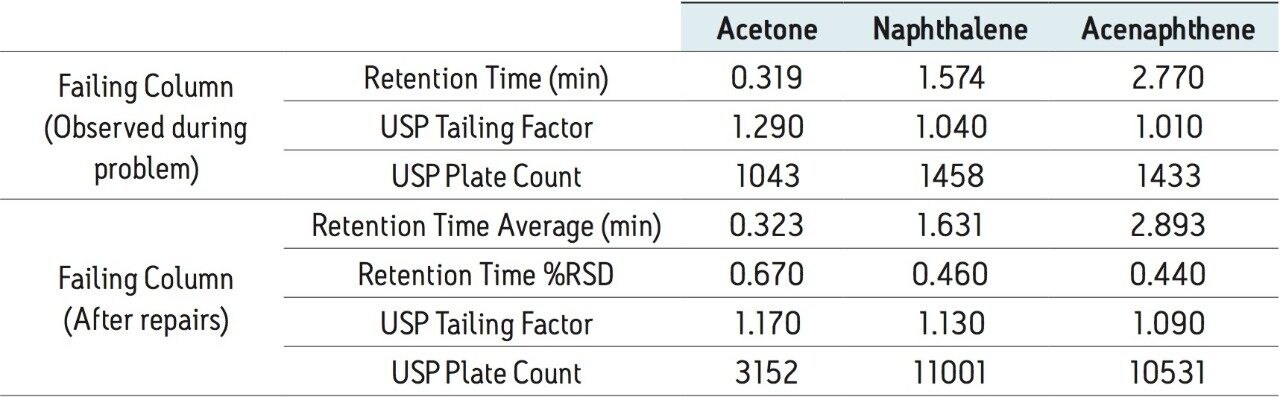
As Figure 1 shows, the failing column is causing peak splitting of both the naphthalene and acenaphthene peaks. Monitoring USP plate count for the acenaphthene peak, the value dropped to approximately 1000 with the failing column, shown in Table 3. After the failing column was replaced with a new column, nine injections of the Neutrals QCRM were run. The data from these nine injections, shown in Table 3, is comparable to the benchmarked data, indicating that the system is operating optimally prior to column failure. The low %RSD of the combined retention times after column replacement, as well as the consistent return to comparable plate counts and tailing factors, demonstrate that the system is back to normal performance.
The second system issue demonstrated is malfunctioning pump caused by a leak. Once the leak was induced, the Neutrals QCRM was analyzed, shown in Figure 2.
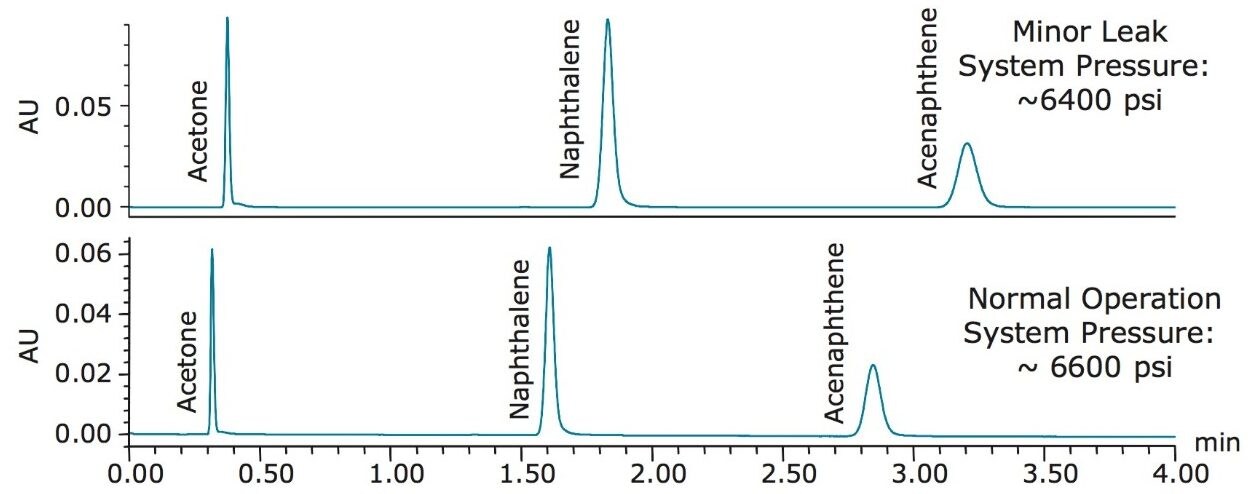
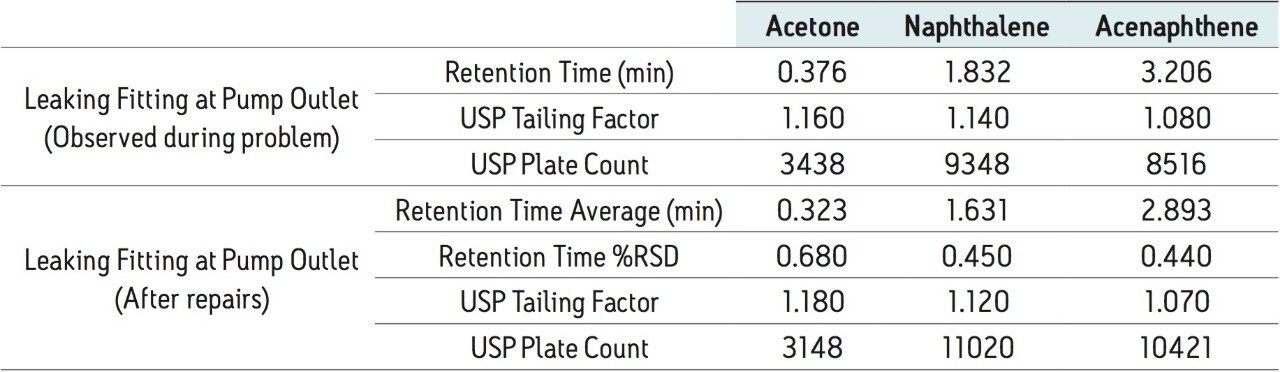
When a minor leak was present in the pump, all of the peaks were still eluting within the sample run time; however, there is a shift in retention times and a slight change in the system pressure. With stringent specifications set in the laboratory, the approximate 10% difference in retention times, shown in Table 4, may fall outside of the specifications, alerting the analyst of a potential system issue. Combined with the pressure difference, this may indicate to the analyst that the pump could be malfunctioning. After the pump was repaired, the system was re-checked for performance using the Neutrals QCRM. Nine injections of the standard were performed and the data was compiled, shown in Table 4. The combined retention time %RSDs were less than 0.7 for all peaks after the leak was repaired, which confirms that the system was back to normal operation.
A third common system problem is a bad check valve. Check valves help to regulate flow and pressure in an HPLC/UPLC system. Over time, these valves may stick and become clogged depending on the types of mobile phase used. When they begin to fail, there can be noticeable chromatographic and pressure issues in a system. The separation of the Neutrals QCRM on a system with a bad check valve compared to a good check valve is shown in Figure 3.
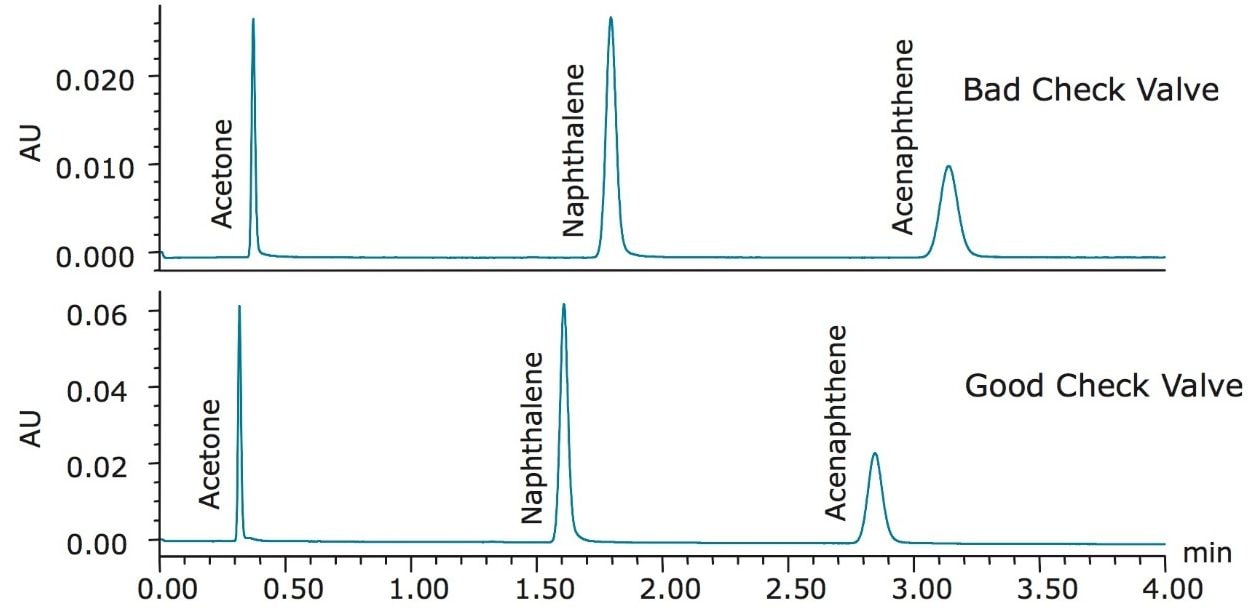
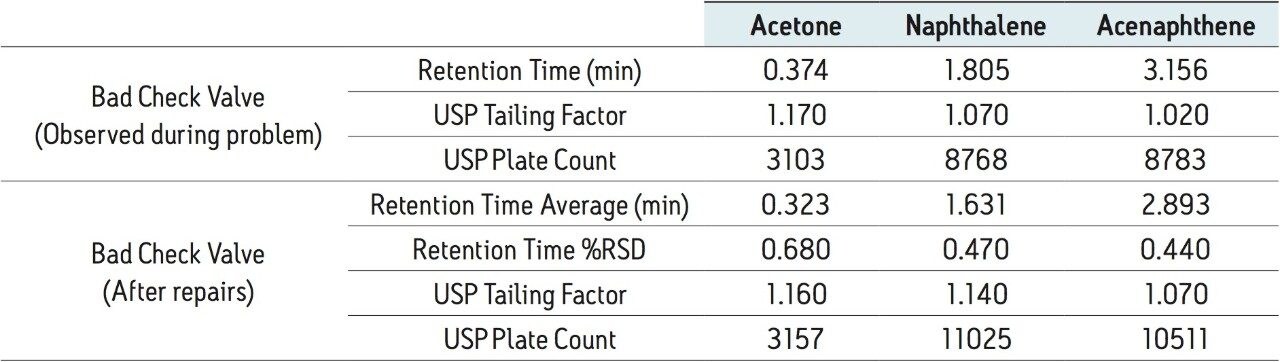
The retention times of all three peaks shift with the bad check valve compared to the good check valve. This slight change in retention time is caused by the check valve not being able to regulate the flow of the mobile phase effectively. In this instance, not only did the retention of the compounds increase, but the plate count dropped by 26% for naphthalene, shown in Table 5. This shift in retention time, as well as the decrease in plate count, may cause a run of standard to fall out of specifications. Once the check valve was replaced, the system was checked for performance once again by running an additional nine injections of Neutrals QCRM, shown in Table 5. In this instance, the plate counts and retention times of the nine injections were comparable to the benchmarked data, indicating a normally functioning system after repair of the check valve.
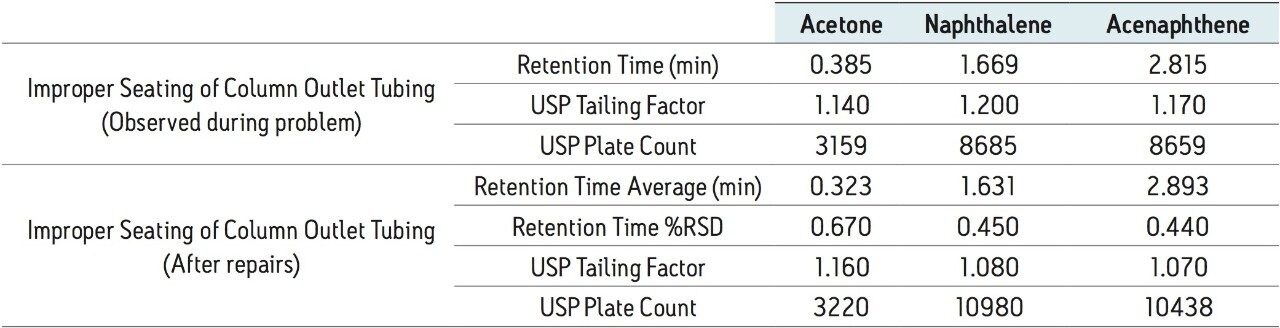
A fourth common mode of failure in an LC system is improper column connection. Improperly connecting the tubing to the column can occur when changing columns and can result in a gap between tubing and column end fittings. This gap can affect peak shape, potentially widening peaks, resulting in excessive peak tailing or shouldering. The separation of the Neutrals QCRM on a column with an improper connection compared to a column that is properly connected can be seen in Figure 4.
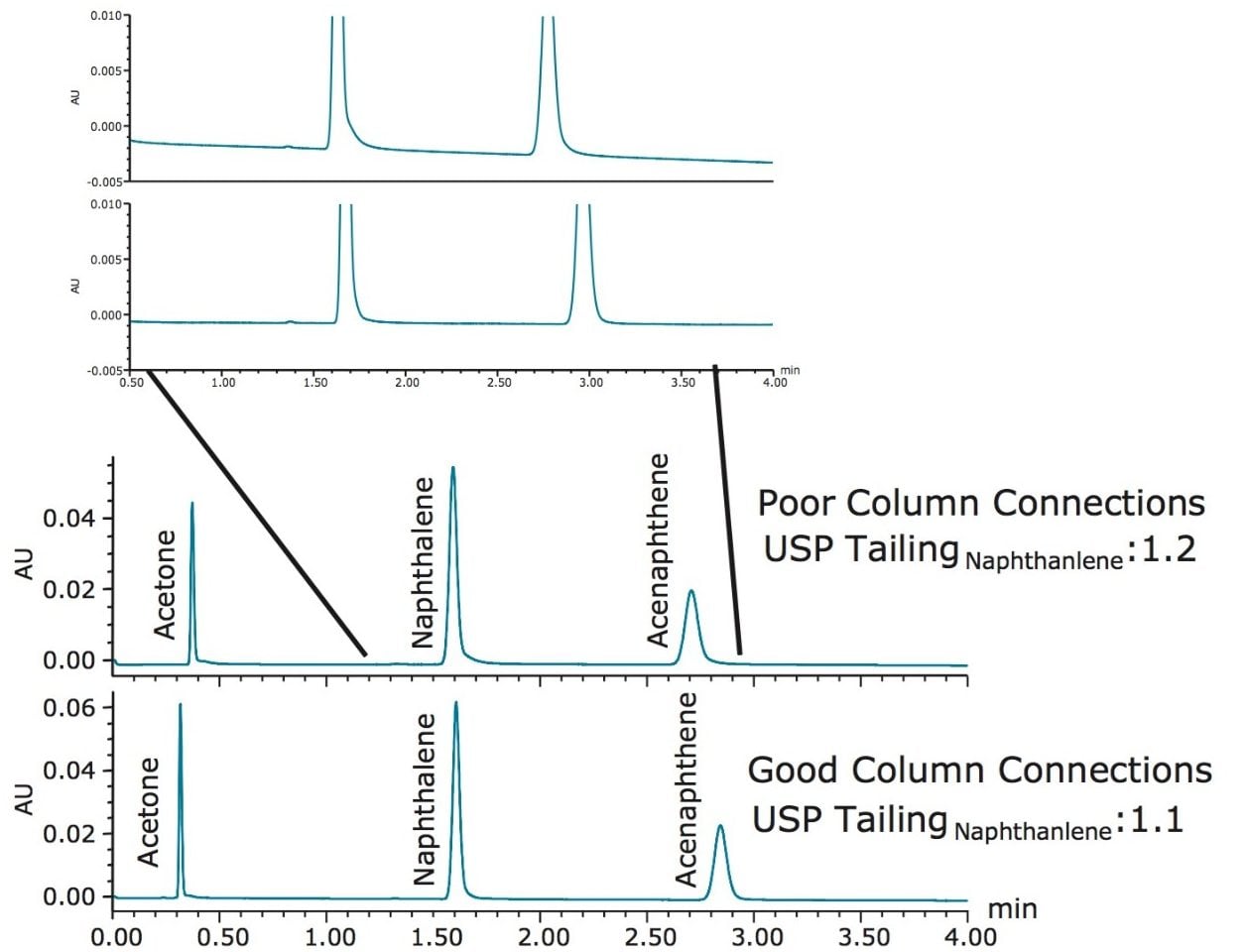
In this example, the separation of the Neutrals QCRM with the improper column connection shows only slight changes in the separation compared to the proper column connection. The effect of improper column connections can vary depending on the extent of the gap created. In this case, tailing of the naphthalene peak increases slightly with the poor tubing installation as well as the decreased retention of the acenaphthene peak. These changes could indicate many problems with the system. Each peak is affected differently and as the differences are only slight, they might go unnoticed. In addition to the higher tailing of the naphthalene peak, a drop in plate count is also observed, shown in Table 6. Depending on assays and specifications, this drop in plate count may cause system performance checks to fail. By connecting the column properly and injecting nine injections of the standard, the system performance was re-checked, shown in Table 6. The tailing factor for naphthalene returned to approximately 1.1, and the plate counts increased and returned to the same performance as the benchmarked data, indicating that the system has returned to optimal operation.
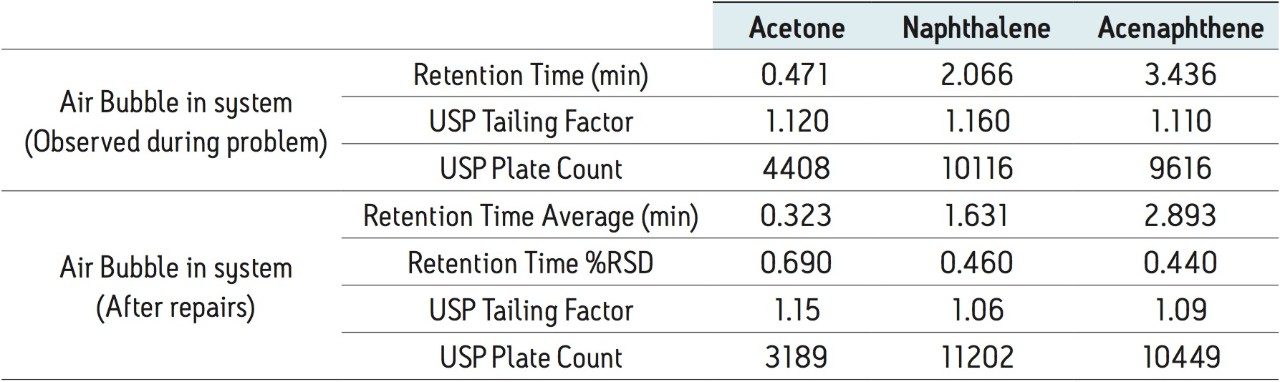
A fifth common problem in LC is an air bubble in the solvent line, which can be caused by inadequate system priming or running out of solvent in the solvent bottles. Once an air bubble forms, it can affect the system pressure and mobile phase delivery. The effect of an air bubble on the separation of the Neutrals QCRM is shown in Figure 5.
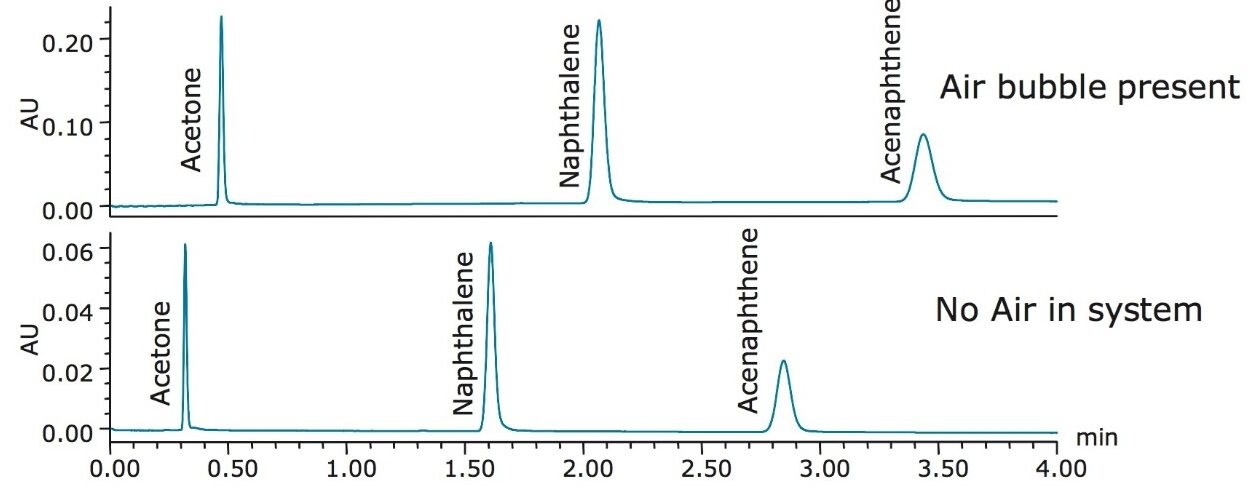
With air in the system, the retention time of all the peaks has shifted. The air in the solvent lines or pump can cause improper delivery of the mobile phase, thereby shifting retention time. In this case, a 25% increase in retention of the naphthalene peak was observed, shown in Table 7. By re-priming the system with mobile phase, the air was removed from the system. Looking at the data from nine injections of the Neutrals QCRM after removing air from the system, shown in Table 7, the retention times have returned to where they were during system benchmarking, indicating the system no longer has an air bubble.
The final common problem seen in LC that was studied in this application is varying organic composition in the mobile phase, which can happen during mobile phase preparation due to analyst error. Slight variations in mobile phase composition can have effects on chromatographic results, including increasing or decreasing retention times and potentially causing co-elution of peaks. In this application, the percentage of acetonitrile was altered by ± 2% for the analysis of the Neutrals QCRM. Figure 6 shows the separation with using mobile phase compositions of 48%, 52%, and 50% (recommended composition) acetonitrile.
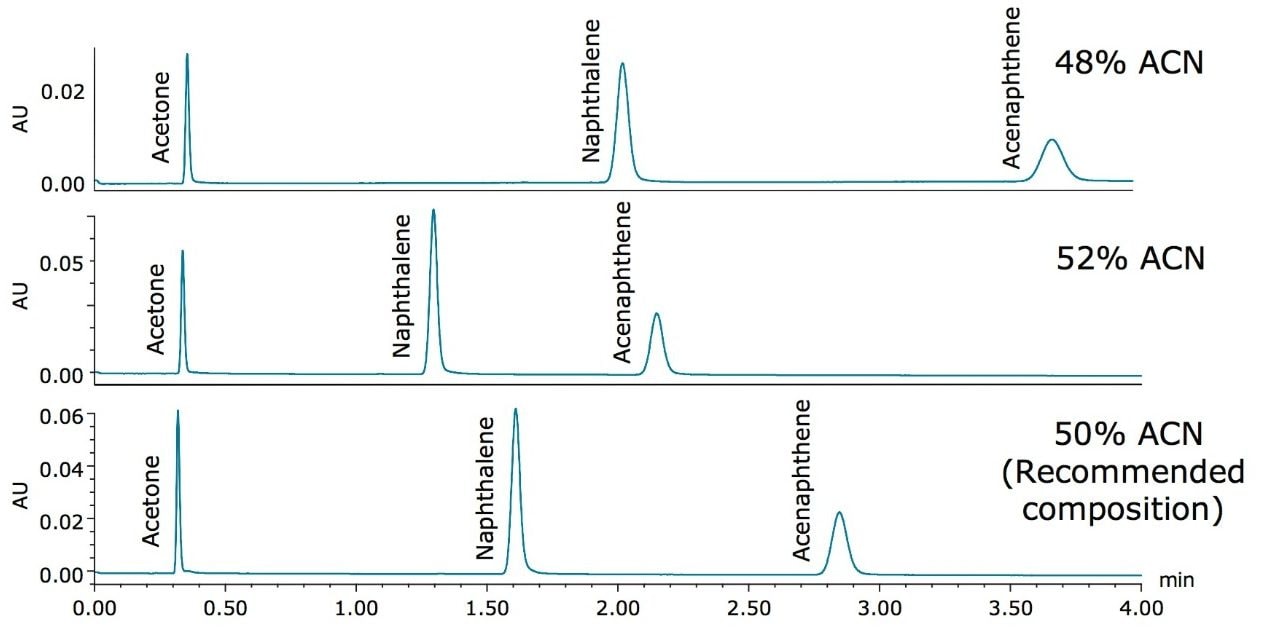
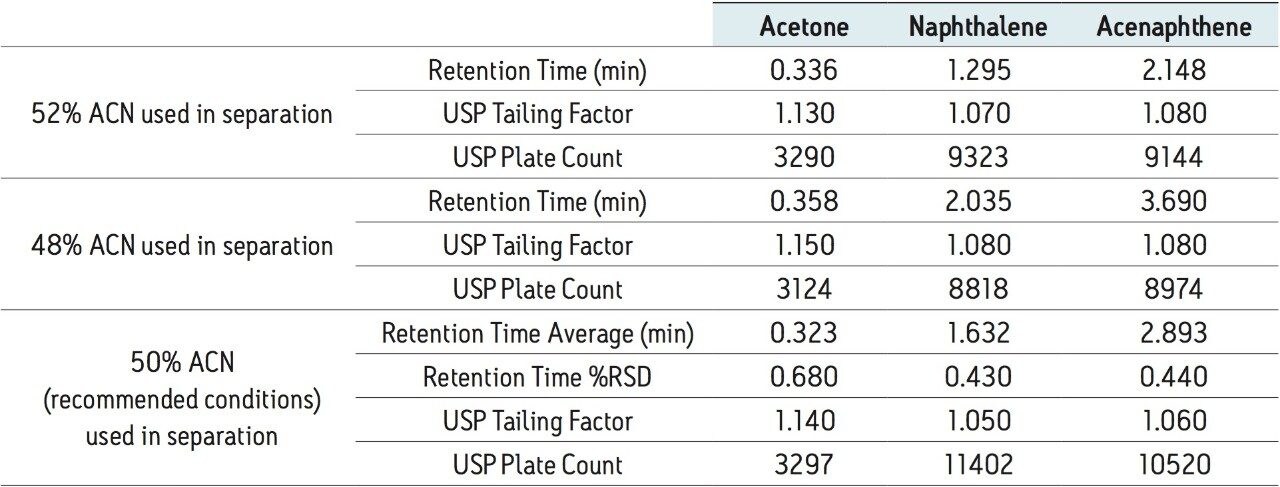
Predictably, the varying organic composition has a significant effect on the isocratic separation of the standard. A retention time shift of 25% for the naphthalene peak was observed when the mobile phase contained 48% acetonitrile, while a 21% decrease in retention time occurred when 52% acetonitrile was present in the mobile phase, shown in Table 8. Once the original mobile phase composition (50% acetonitrile) was placed back onto the system, nine injections of the Neutrals QCRM were run to re-check the system performance, shown in Table 8, and to demonstrate proper system operation. The retention times of all the peaks were comparable to the benchmarked data, indicating that the mobile phase was made accurately and that the system is functioning as it should. The Neutrals QCRM is compatible with many mobile phases, and if the mobile phases for sample analysis are used to both benchmark and troubleshoot the system, the benefits of the Neutrals QCRM for troubleshooting mobile phase errors can be realized. While this application focuses on the use of 50% acetonitrile, other mobile phases with sufficient organic composition may be used. Without the use of a standard to check system performance, an error in mobile phase preparation could cause irreproducible chromatography or co-elution of target peaks in real samples, resulting in extensive and unnecessary method development, or reanalysis of the samples. Instead, with proper specifications for the Neutrals QCRM, errors in organic composition may be identified before time is invested in sample analysis.
The Waters Neutrals QC Reference Material (QCRM) is a mixture of three neutral compounds that can be separated with common mobile phases with sufficient organic composition, and is compatible with most column chemistries, making it an ideal standard for troubleshooting HPLC and UPLC system problems. Before the standard can be used as a troubleshooting tool, it is recommended to benchmark the system performance using the standard and create a set of specifications to determine the limits of acceptable data for future runs of the standard.1 During routine analysis the standard can be used to monitor to the system and if a problem arises, the Neutrals QCRM can be run to determine if a system problem exists and to help identify the issue. Once the problem is resolved, the standard can be run again to confirm that the system is back to normal operation.
There are many benefits to using the Neutrals QCRM. First, system downtime can be reduced. This allows for a better use of resources, with more samples analyzed and less time spent by analysts trying to fix a system problem. Secondly, as a result of the strict manufacturing process of the standard, errors in suitability standard preparation are mitigated, isolating issues to the system and allowing a chromatographer to identify the problem faster. Lastly, the Neutrals QCRM can be used to ensure that a recently repaired system is functioning optimally. This increases the confidence in the data produced after repairs and ensures high quality data generation.
720004635, March 2013