This is an Application Brief and does not contain a detailed Experimental section.
A significant number of tandem quadrupole mass spectrometers are employed for the quantification of trace compounds found in complex biological samples, such as plasma, urine, etc. Critical elements within the instrument can be sensitive to contamination by deposits from these complex matrices resulting in a progressive loss in instrument performance. The Waters Micromass Quattro Premier benchtop mass spectrometer is equipped with a new and improved ZSpray atmospheric pressure ionization LC interface. This source design safeguards critical source and analyzer elements against the harmful effects of contamination by complex (dirty) sample matrices. The Quattro Premier provides robust MRM data for quantification even when using difficult matrices.
Safeguards critical source and analyzer elements against the harmful effects of contamination by complex (dirty) sample matrices.
A significant number of tandem quadrupole mass spectrometers are employed for the quantification of trace compounds found in complex biological samples, such as plasma, urine, etc. Critical elements within the instrument can be sensitive to contamination by deposits from these complex matrices resulting in a progressive loss in instrument performance. The Waters Micromass Quattro Premier benchtop mass spectrometer is equipped with a new and improved ZSpray atmospheric pressure ionization LC interface. This source design safeguards critical source and analyzer elements against the harmful effects of contamination by complex (dirty) sample matrices. The Quattro Premier provides robust MRM data for quantification even when using difficult matrices.
The nebulized spray is positioned vertically, with the sprayer orientated orthogonally and positioned off axis for maximum source longevity. An isolation valve also allows the sample cone to be quickly and easily removed for cleaning without breaking the vacuum. A new feature of the Quattro Premier ZSpray source is an exhaust trap. This trap is positioned directly opposite the ionization probe. The flow path of nitrogen within the ionization chamber ensures that non-ionized materials entering the source are rapidly removed. Like the sample cone this trap can be easily removed for cleaning.
Human plasma was protein precipitated by the addition of acetonitrile (2:1) and then spiked with verapamil. To evaluate the robustness of the Quattro Premier mass spectrometer ionization source over 200 10 μL injections of this sample were analyzed by LC-MS/MS. The study ran over a 24-hour period in positive ion electrospray ionization mode (ESI+ve).
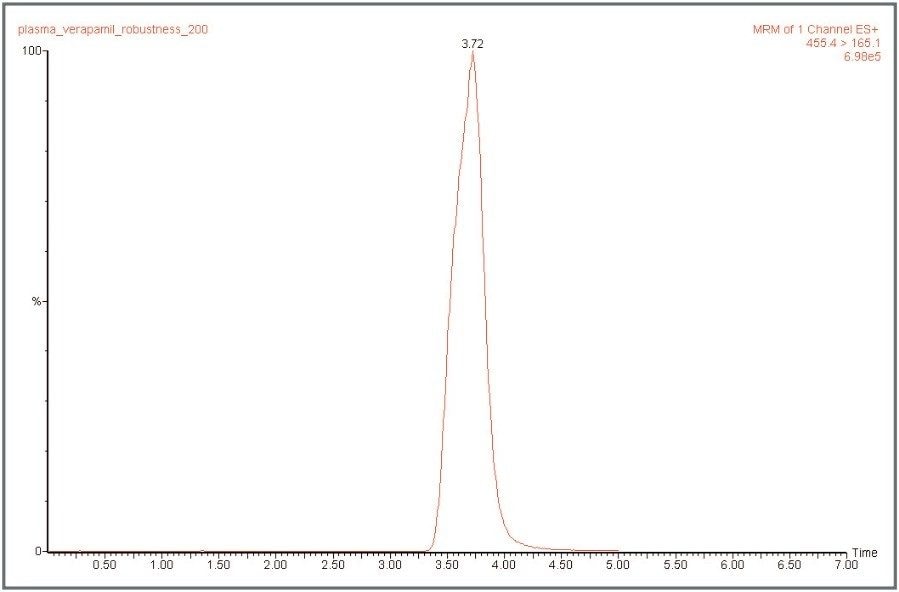
HPLC separation (Figure 1) was performed using a Waters Atlantis Column (2.1 x 100 mm, C18, 3.5 μm) using isocratic mobile phase conditions (55% H20/, 45% ACN, 0.1% formic acid, flowing at 200 μL/min). The LC flow was delivered directly to the electrospray ion source of the MS. No flow diversion or flow splitting was used. MS data acquisition was performed in the multiple reactionmonitoring mode (MRM) using the parent ion to product ion transition of m/z 455 to m/z 165.
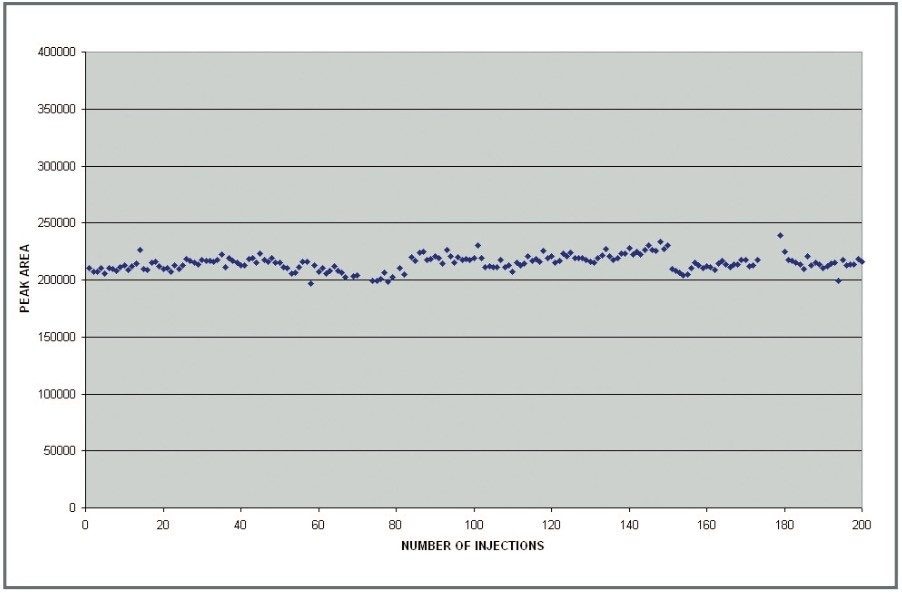
The relative standard deviation (%CV) for all 200 peak areas presented in Figure 2 was 3%. This demonstrates that the Quattro Premier mass spectrometer provides robust MRM data for reliable and reproducible quantification even when analyzing compounds in complex (dirty) matrices.
720000916, June 2004