
In this application note, example performance data is provided from Waters ACQUITY UPLC I-Class System and Xevo TQ-S micro on three commodities which represent high water content and high starch, low water content sample types. The aim of this study was to evaluate the combination of the ACQUITY UPLC I-Class System coupled with Xevo TQ-S micro for the determination of highly polar cationic pesticide residues and plant growth regulators in several food commodities.
Provides a direct, single extraction LC-MS/MS method for the analysis of various highly polar cationic pesticides and plant growth regulators in cereals, fruit, and vegetable commodities.
The European Union Reference Laboratory for Pesticides Single Residue Methods (EURL-SRM) published the QuPPe (Quick Polar Pesticides)1 methods for the simultaneous analysis of a number of highly polar pesticides. To meet the needs of analyzing highly polar pesticides by LC-MS/MS, details on a number of chromatographic methods have been provided including one based upon hydrophilic interaction liquid chromatography (HILIC)1-2 for the determination of a series of cationic and polar basic analytes.
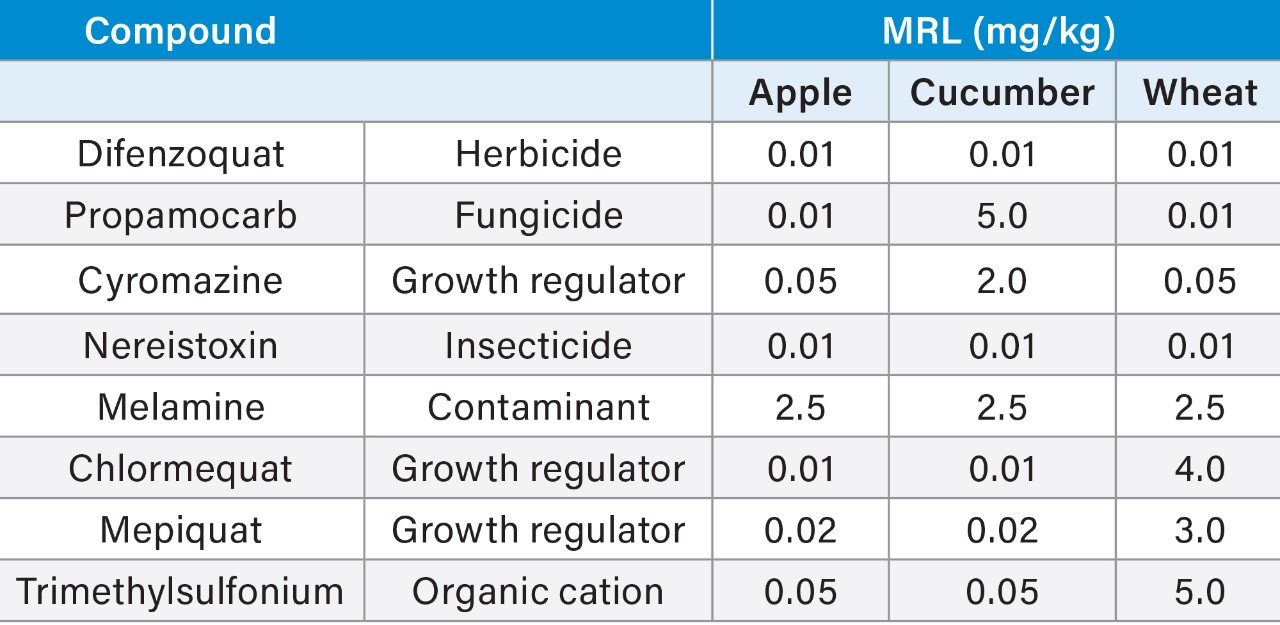
Although several compounds included in this application note are approved for use in Europe (maximum residue limits (MRLs) are listed in Table 1), other pesticide/crop combinations are not and default MRLs apply.3 As well as being a separate contaminant of interest, melamine4 is also a metabolite of cyromazine, although it is not yet part of the residue definition used for enforcement purposes.
In this application note, example performance data is provided from Waters ACQUITY UPLC I-Class System and Xevo TQ-S micro on three commodities which represent high water content and high starch, low water content sample types. Organic wheat flour, cucumber, and apple, were extracted following the QuPPe method,1 to assess various performance factors of the UPLC-MS/MS method such as calibration linearity, retention time stability, method precision, and trueness.
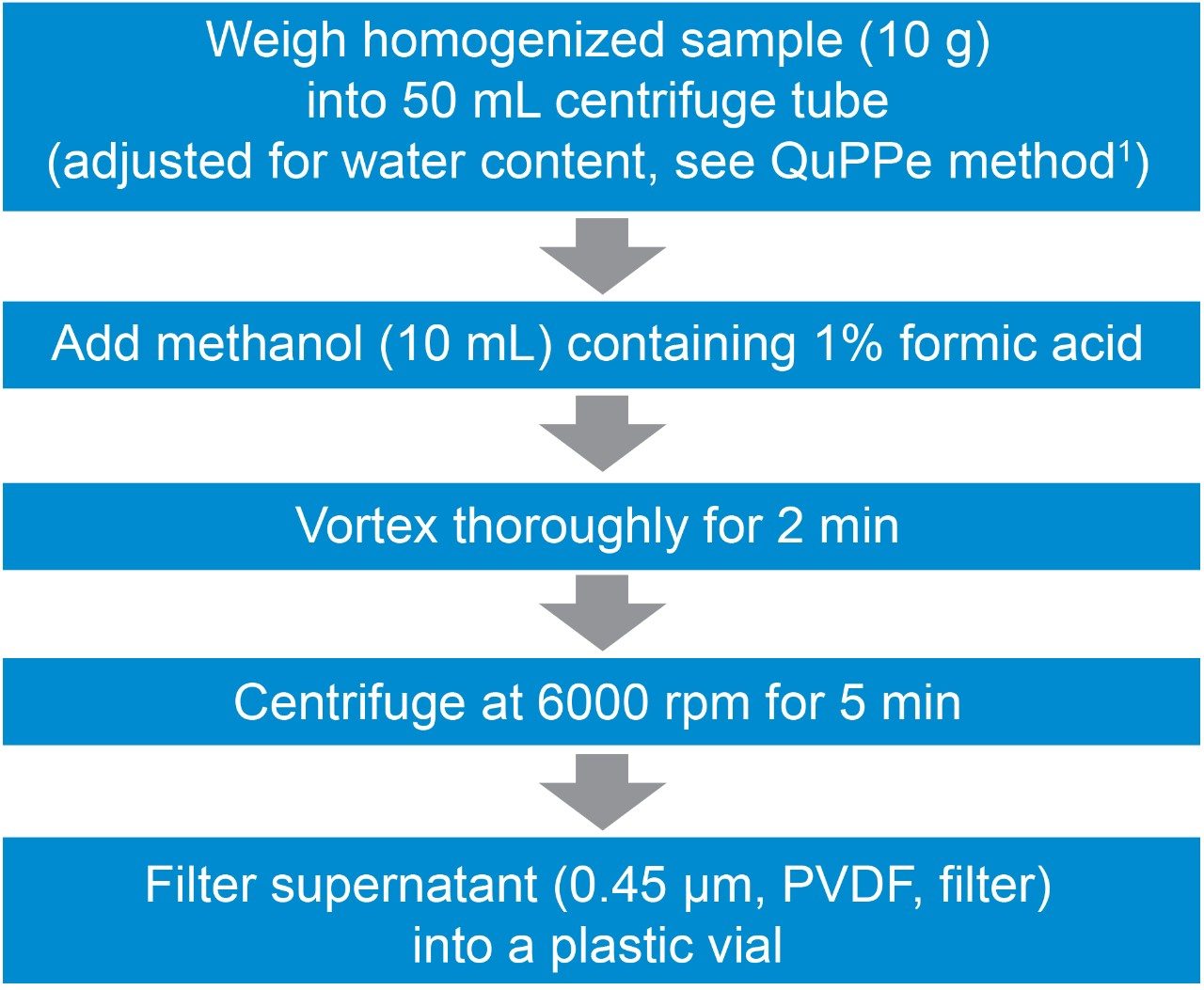
Homogenized organic apple and cucumber were extracted using the QuPPe method1 as shown in Figure 1. For wheat flour only 5 g of sample was taken and 10 mL of LCMS grade water was added to this before extraction with the acidified methanol. Before the centrifugation step, the wheat flour was placed in a freezer at -20 °C for 2 hrs. The supernatant from the QuPPe extracts were then filtered using a 0.45 µm PVDF filter, spiked with the pesticide mix and analyzed using the liquid chromatography, mass spectrometry method highlighted below. Method performance information for analyte recovery can be found in the QuPPe document.1
The performance of the LC-MS/MS step of the method was assessed using SANTE guidelines.5 Solutions of matrix-matched standards were prepared over the range 0.002 to 0.200 mg/kg (1.0 to 100.0 ng/mL in vial concentration) in apple and cucumber, 0.004 to 0.400 mg/kg (1.0 to 100.0 ng/mL in vial concentration) in wheat flour. Replicate injections at two concentration levels were run between bracketed calibration curves to assess the performance of the method. No isotopically labelled standards were used for this analysis.
|
UPLC system: |
ACQUITY UPLC I-Class with fixed-loop Sample Manager |
|
Column: |
ACQUITY UPLC BEH Amide, 1.7 μm, 2.1 × 100 mm (p/n: 186004801) |
|
Mobile phase A: |
50 mM Ammonium formate (pH 2.9, adjusted with LCMS grade formic acid) |
|
Mobile phase B: |
Acetonitrile |
|
Flow rate: |
0.5 mL/min |
|
Injection volume: |
0.5 μL (partial loop needle overfill) |
|
Weak wash solvent: |
90:10 acetonitrile:water |
|
Strong wash solvent: |
10:90 acetonitrile:water |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Run time: |
10 min |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
0.00 |
3.0 |
97.0 |
Initial |
|
0.50 |
3.0 |
97.0 |
6 |
|
4.00 |
30.0 |
70.0 |
6 |
|
5.00 |
40.0 |
60.0 |
6 |
|
6.00 |
40.0 |
60.0 |
6 |
|
6.10 |
3.0 |
97.0 |
6 |
|
10.00 |
3.0 |
97.0 |
6 |
|
MS system: |
Xevo TQ-S micro |
|
Ionization: |
ESI+ |
|
Capillary voltage: |
0.5 kV |
|
Desolvation temp.: |
600 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/Hr |
|
Nebulizer gas pressure: |
7 Bar |
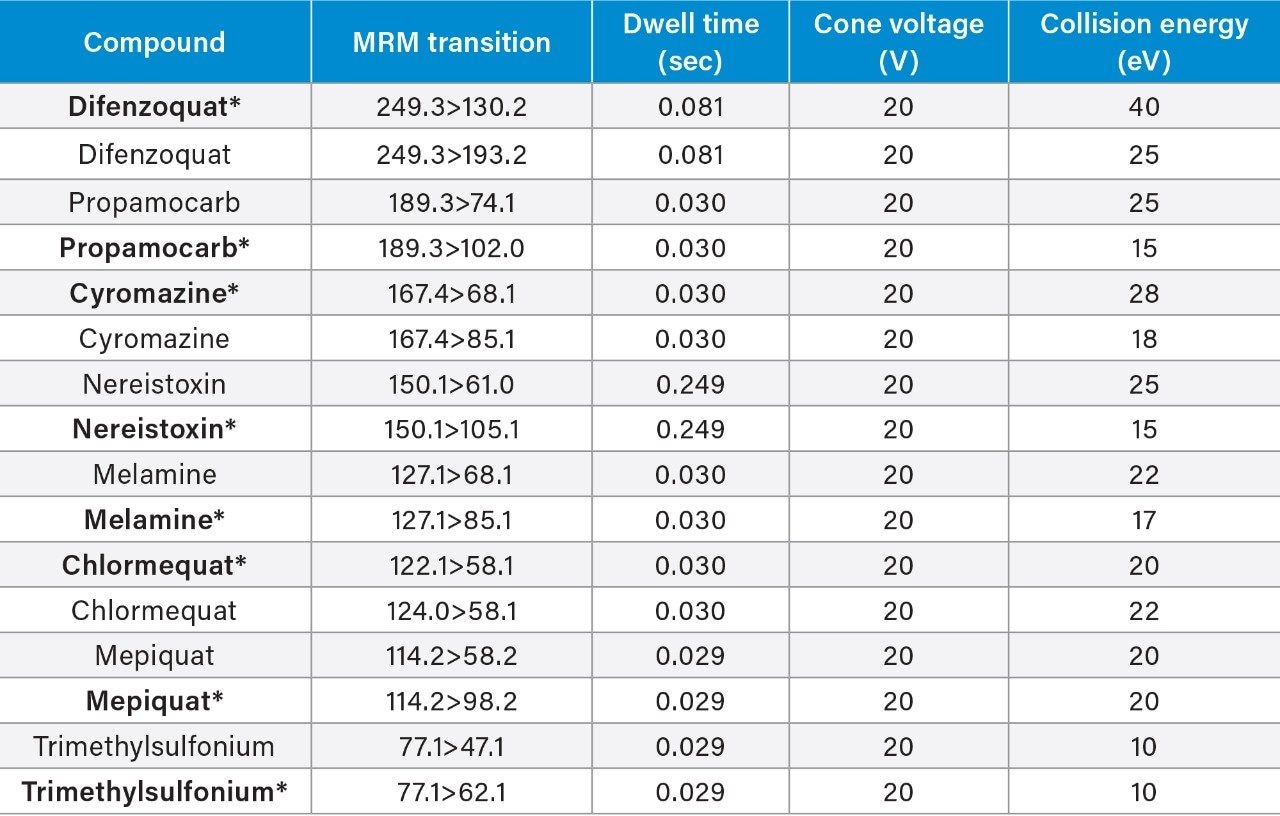
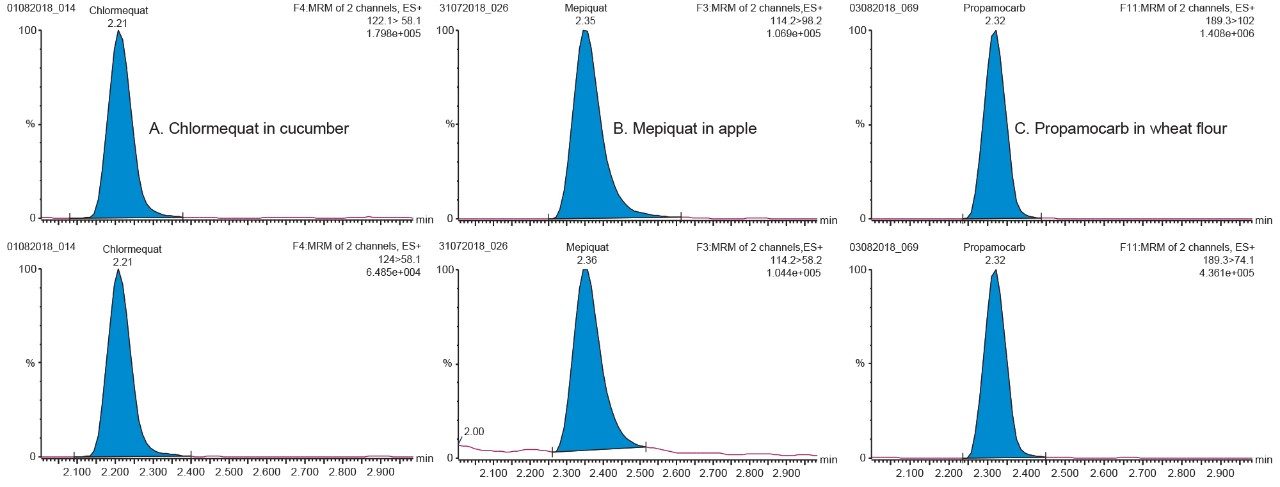
The method was found to give good retention for all compounds, greater than two times the column void volume, as indicated in the SANTE guidelines.5 Overall the method provided acceptable separation and excellent peak shapes for all compounds. Example chromatograms for chlormequat, mepiquat, and propamocarb in matrix at the 1 ng/mL in vial concentration level, are shown in Figure 2. Retention time stability was also assessed according to the SANTE guidelines (±0.1 min),5 retention times within and between matrices were within 0.1 min for all compounds. Retention times for each compound in the representative matrices can be found in Figure 3.
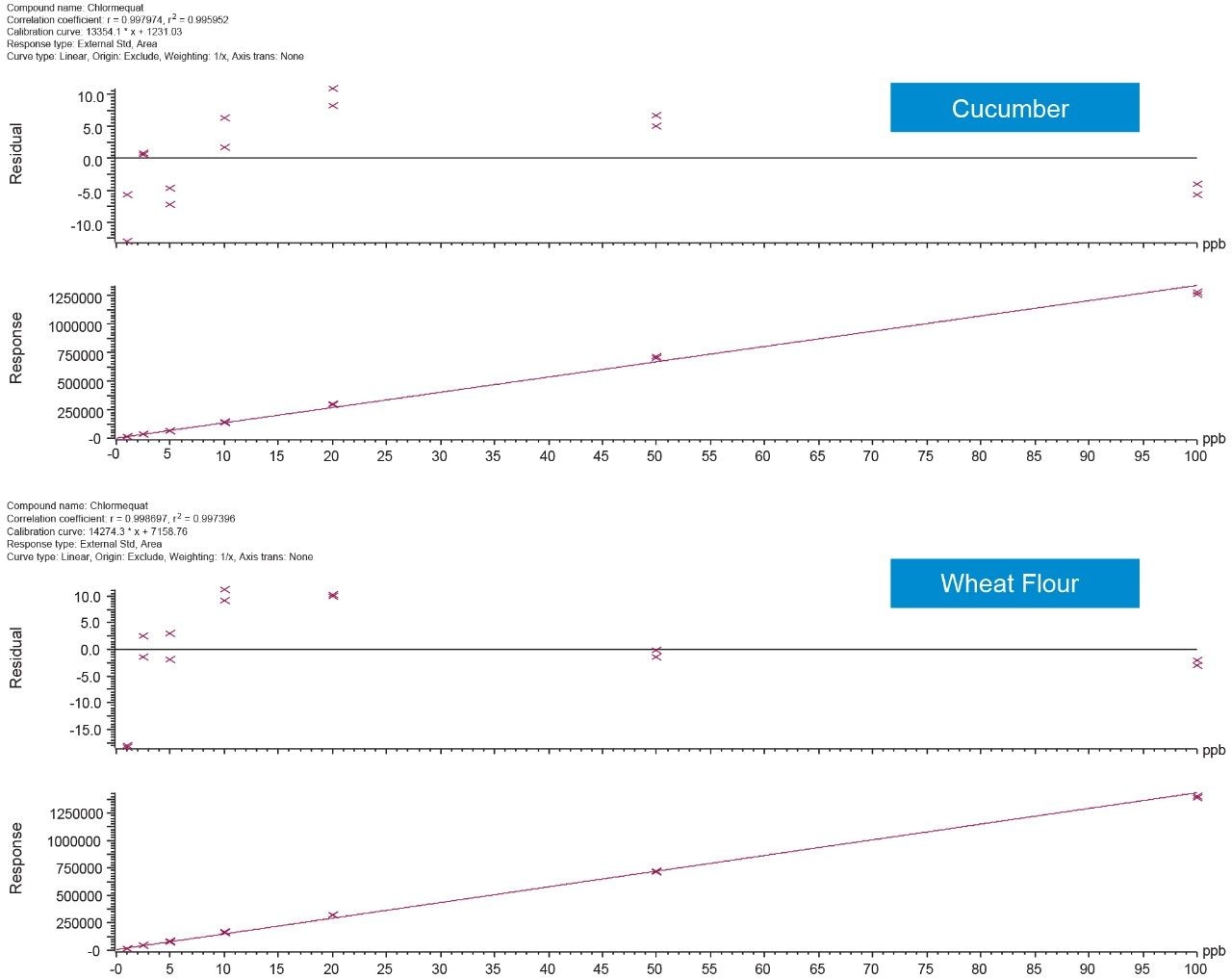
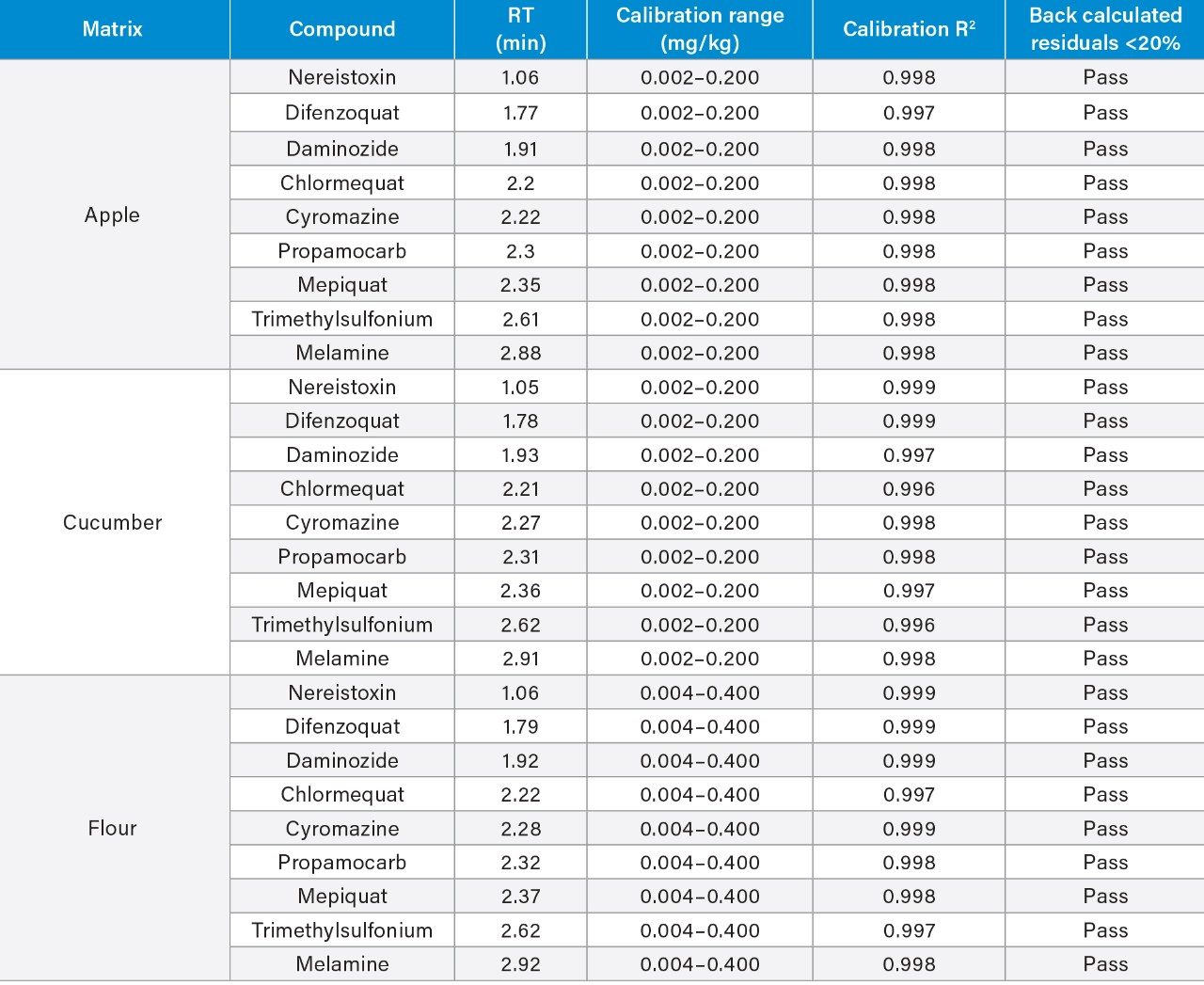
The linearity of the method was assessed using bracketed matrix-matched calibration curves for each matrix, without the use of labeled internal standards; Figure 3 shows the calibration curves for chlormequat in organic cucumber and wheat flour. The linearity of response and calibration range in the tested matrices for all compounds assessed in this study, are shown in Table 2. The concentration levels take into account that only 5 g of wheat flour was taken for extraction. All compounds gave excellent linear response and residuals (back calculated concentrations) were within the 20% tolerance of the SANTE guidelines.5
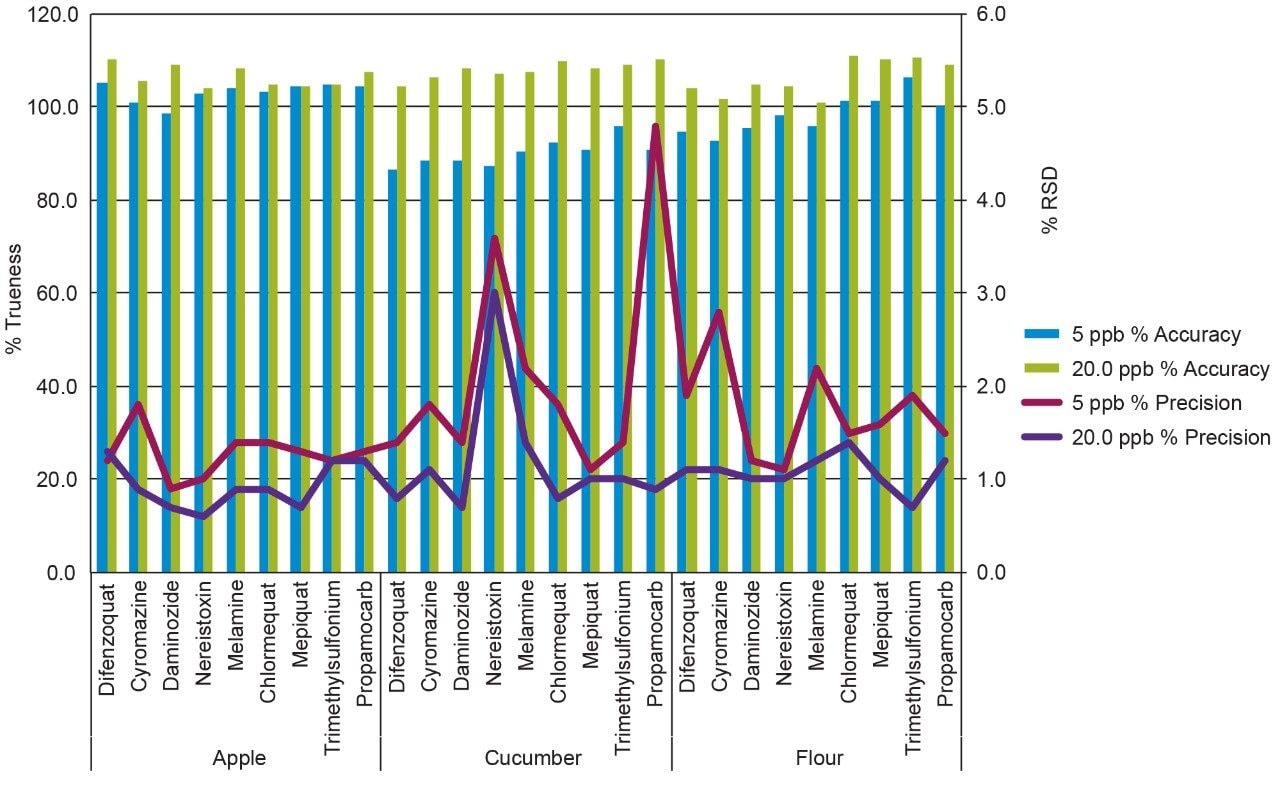
Replicate (n=15) injections were run for two levels, 5.0 ng/mL and 20.0 ng/mL in vial concentrations. The calculated mean concentrations and precision for the tested compounds in all three matrices can be seen in Figure 4. Excellent accuracy and precision was achieved for all compounds, within 15% of the target concentration value and %RSD below 5%.
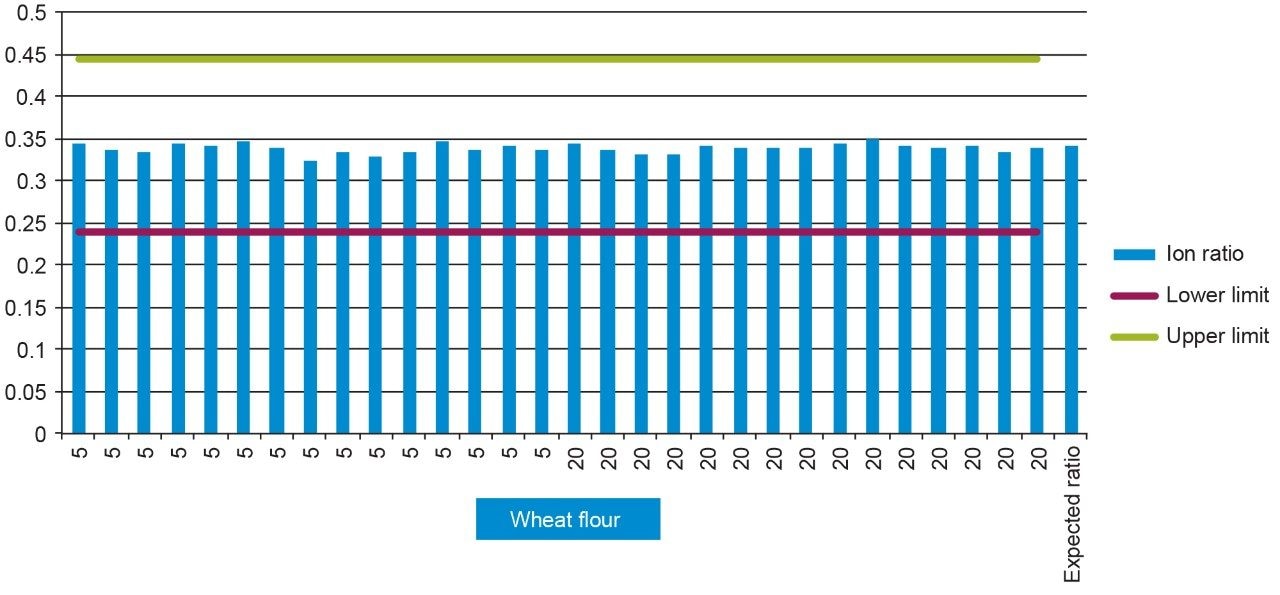
Ion ratios of the replicate injections agreed well with expected reference values and all were within the required tolerance5 (±30%). An example of the ion ratios given by each of the (n=15) replicate levels for chlomequat in wheat flour can be seen in Figure 5.
The aim of this study was to evaluate the combination of the ACQUITY UPLC I-Class System coupled with Xevo TQ-S micro for the determination of highly polar cationic pesticide residues and plant growth regulators in several food commodities. The Xevo TQ-S micro provided excellent, fit-for-purpose performance in terms of sensitivity, linearity, and calibration range for all of the tested matrices. The trueness and precision of this UPLC-MS/MS method determined at two matrix QC levels with 15 replicate injections was found to be acceptable for all compounds. Overall the performance data indicate that the configuration of the ACQUITY UPLC I-Class coupled with Xevo TQ-S micro, when used in combination with the ACQUITY UPLC BEH Amide Column and an established extraction protocol such as QuPPe, is suitable for checking MRL/tolerance compliance in routine laboratory testing for these target compounds.
720006410, October 2018