For forensic toxicology use only.
A novel reversed-phase SPE sorbent is described in this application note, Oasis PRiME has been used to achieve consistent, high recoveries of synthetic cannabinoids and metabolites with low matrix effects while virtually eliminating endogenous phospholipids from whole blood samples. The enabled excellent quantitiative results, even without the use of deuterated internal standards.
Oasis PRiME HLB is a novel reversed phase solid phase extraction (SPE) sorbent developed to enable simpler and faster SPE protocols, while at the same time generating cleaner extracts than other sample preparation methods. With Oasis PRiME HLB, a 3-step load-wash-elute SPE protocol eliminating conditioning and equilibration was successfully employed to extract 22 synthetic cannabinoids and metabolites from whole blood samples. Excellent analyte recoveries and modest matrix effects (ME) were achieved across the entire panel of compounds. These results were consistent, with low variability for all compounds. In addition, Oasis PRiME HLB removed more than 95% phospholipids from the whole blood samples compared to protein precipitation (PPT). The 22 synthetic cannabinoids and metabolites were extracted using Waters Oasis PRiME HLB 30 mg plates. Calibration curves for all compounds ranged from 0.2–100 ng/mL. Quantitative results from quality control samples were accurate and precise across the entire calibration range. The analysis of several different classes of these drugs and metabolites, which includes neutral molecules, acids and bases, demonstrates the utility of this method across the different chemotypes and should render this method applicable to newly developed related compounds with little, if any, modification necessary.
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
CORTECS UPLC C18, 90Å, 1.6 μm; 2.1 x 100 mm (p/n 186007095) |
|
Column temp.: |
30 °C |
|
Injection volume: |
5 μL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
0.1% formic acid in MilliQ water |
|
Mobile phase B: |
0.1% formic acid in ACN |
|
Gradient: |
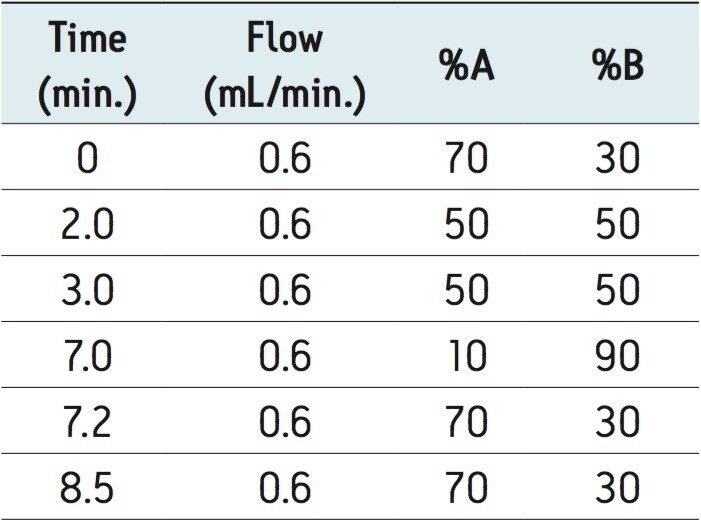
Initial conditions started at 30% B. The %B was increased to 50% over 2 minutes, and held at 50% B for 1 minute, increased to 90% B over 4 minutes and then returned to 30% over 0.2 minutes. The system was allowed to re-equilibrate for 1.3 min. The entire cycle time was 8.5 min. The solvent gradient is listed in Table 1. |
|
MS system: |
Xevo TQD |
|
Ionization mode: |
ESI Positive |
|
Acquisition mode: |
MRM (See Tables 2 and 3 for transitions) |
|
Capillary voltage: |
1 kV |
|
Collision energy (eV): |
Optimized for individual compounds (See Table 2) |
|
Cone voltage (V): |
Optimized for individual compounds (See Table 2) |
All data were acquired and analyzed using Waters MassLynx software v.4.1 (scn 855) and quantified using TargetLynx Software. MS conditions were optimized using Intellistart.
AM2233, JWH-015, RCS-4, JWH-203, RCS-8, JWH-210, JWH-073, and JWH-018 were purchased from Cerilliant (Round Rock, TX). All other compounds and metabolites were purchased from Cayman Chemical (Ann Arbor, MI)
Individual stocks (1 mg/mL) were prepared in methanol, DMSO, or 50:50 DMSO: methanol. A combined stock solution of all compounds (10 μg/mL) was prepared in methanol. Working solutions were prepared daily in 40% methanol.
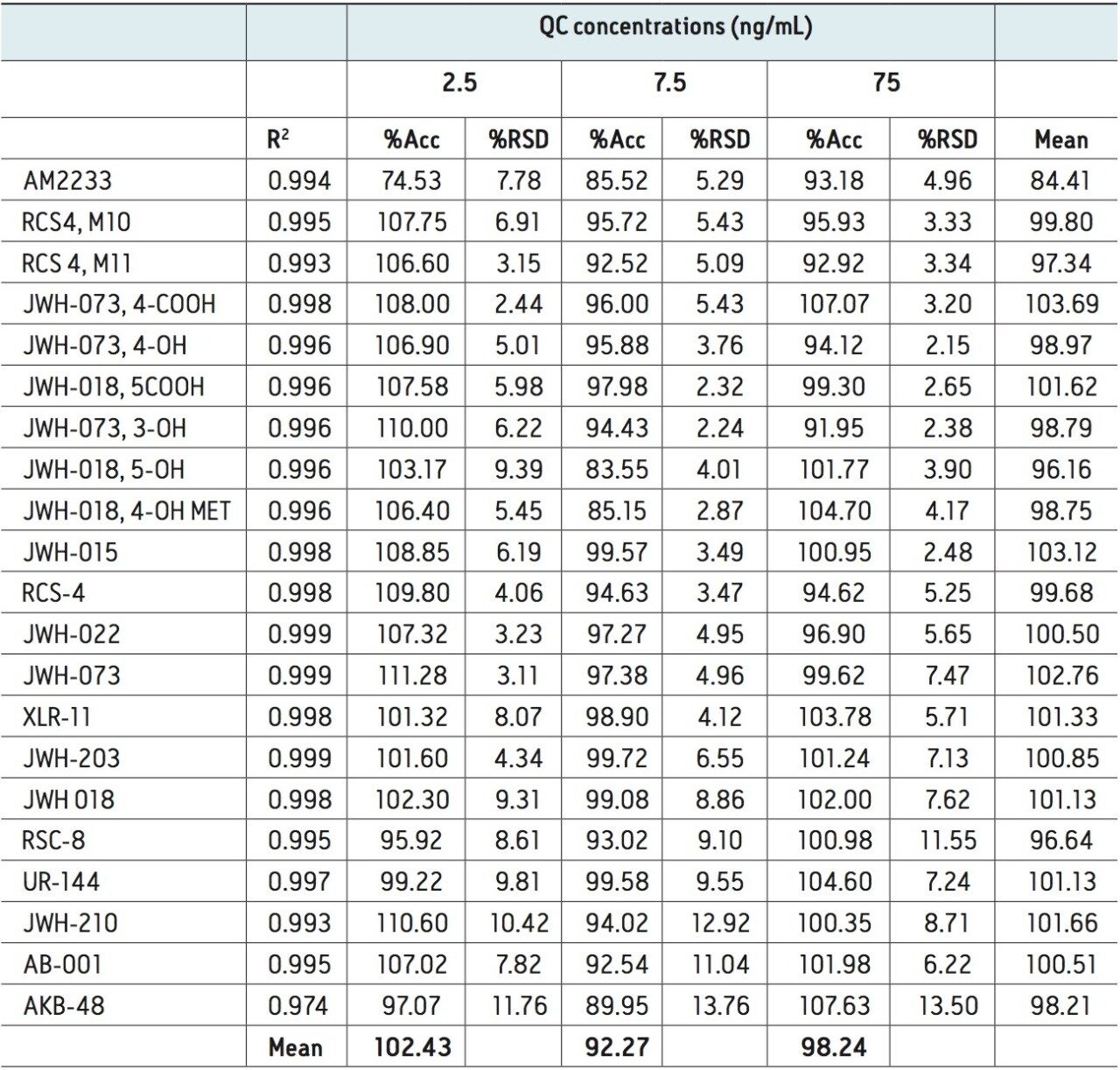
Calibrators and quality control (QC) samples were prepared by spiking working standards at various concentrations and into matrix (whole blood). Calibrator concentrations ranged from 0.2–100 ng/mL for all analytes. Quality control samples were prepared at 2.5, 7.5, and 75 ng/mL, in whole blood.
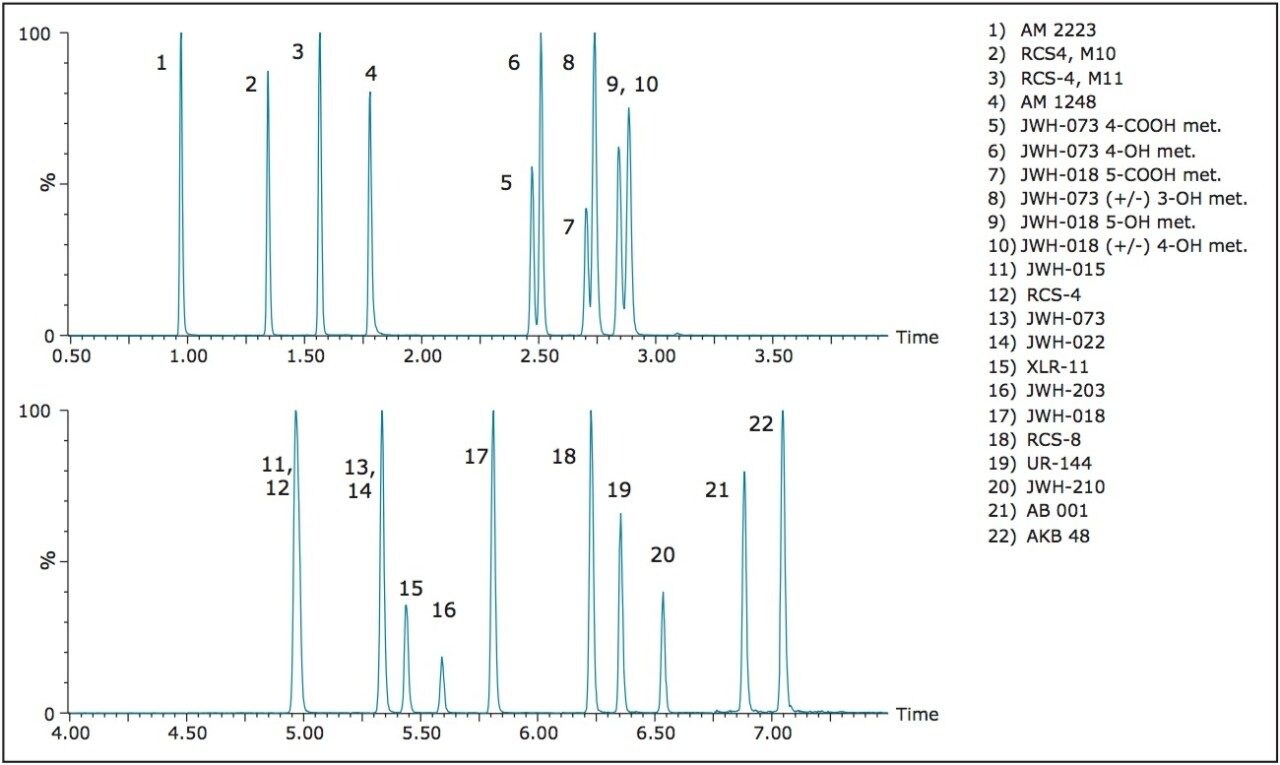
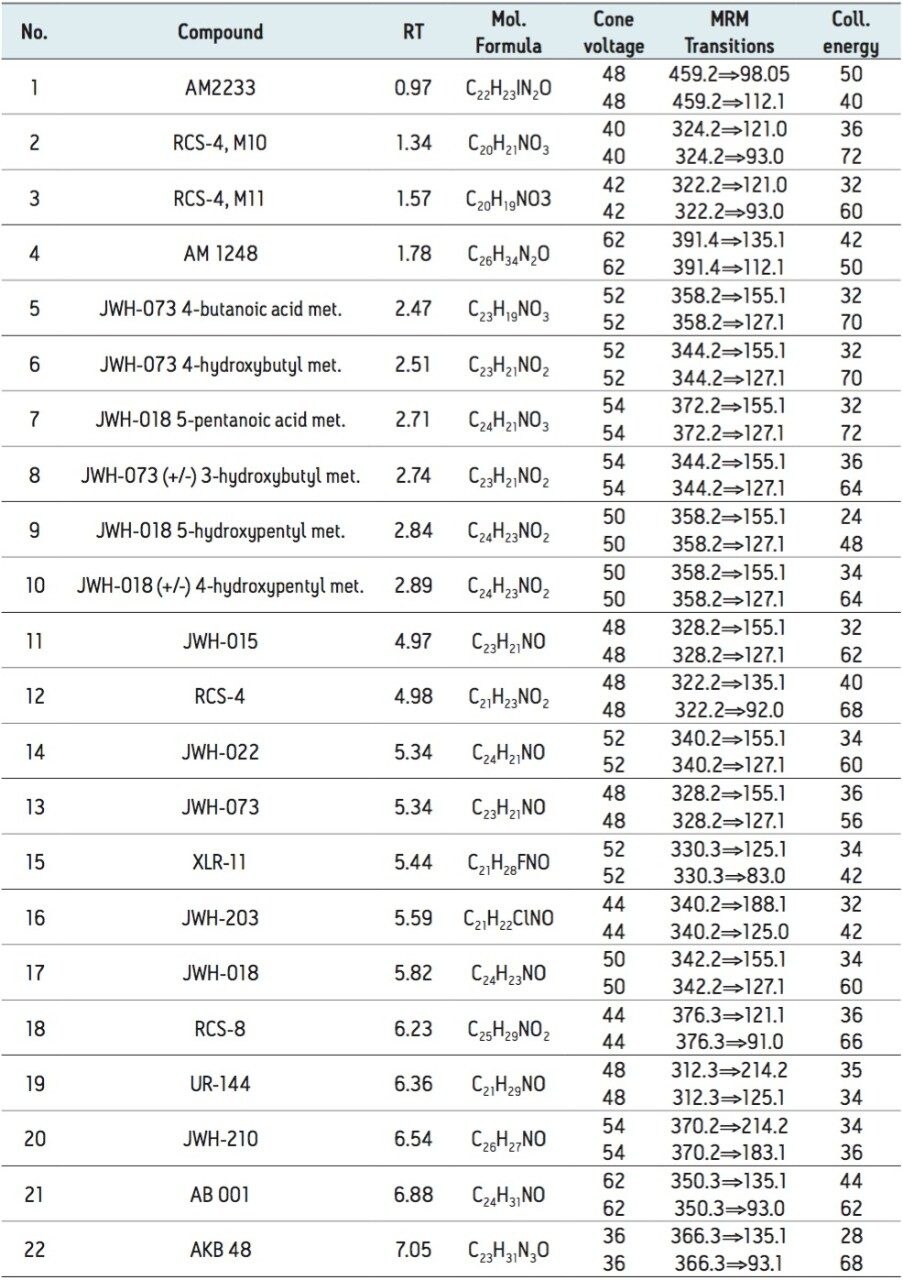
The 22 compounds analyzed are listed in Table 1 and constitute a panel that includes various classes of forensically relevant synthetic cannabinoids. These include adamantoylindoles (AM 1248 and AKB48), napthoylindoles (JWH 022), phenylacetyl indoles (RCS-4 and RCS-8), and tetramethylcyclopropylindoles (UR-144 and XLR11). Major metabolites of JWH-073 and JWH-018 were also included, as some of these compounds are structural isomers with identical mass spectral fragments that require adequate chromatographic separation for accurate quantitation.
Samples were extracted using Oasis PRiME HLB 30 mg Plates. 0.1 mL of a solution of 0.1 M zinc sulfate/ammonium acetate was added to 0.1 mL whole blood, and vortexed for 5 seconds to lyse the cells. All samples were then precipitated by adding 400 μL ACN. The entire sample was vortexed for 10 seconds and centrifuged for 5 min at 7000 rcf. The supernatant was then diluted with 1.2 mL water prior to loading. The sample was directly loaded on the Oasis PRiME 30 mg Plate without conditioning or equilibration. All wells were then washed with 2 x 500 μL 25:75 MeOH:water, and eluted with 2 x 500 μL 90/10 ACN/MeOH. The eluate was then evaporated under Nitrogen and reconstituted with 100 μL 30% ACN. 5 μL was injected onto the UPLC system.
Analyte recovery was calculated according to the following equation:
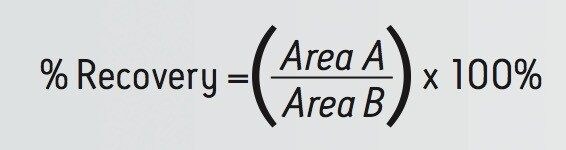
Where A equals the peak area of an extracted sample and B equals the peak area of an extracted matrix sample in which the compounds were added post-extraction.
Matrix effects were calculated according to the following equation:

The peak area in the presence of matrix refers to the peak area of an extracted matrix sample in which the compounds were added post-extraction. The peak area in the absence of matrix refers to analytes in a neat solvent solution.
The design of the solid-core CORTECS Particle, combined with optimal packing in the column, results in excellent chromatographic performance. A representative chromatogram of all compounds from a 20 ng/mL calibration standard is shown in Figure 1. Using a CORTECS UPLC C18 Column (90Å, 1.6 μm, 2.1 x 100 mm), all analytes were analyzed within 7.5 minutes with a total cycle time of 8.5 minutes. Peak shape was excellent for all compounds, with no significant tailing or asymmetries, and all peak widths were under 3 seconds at 5% of baseline.
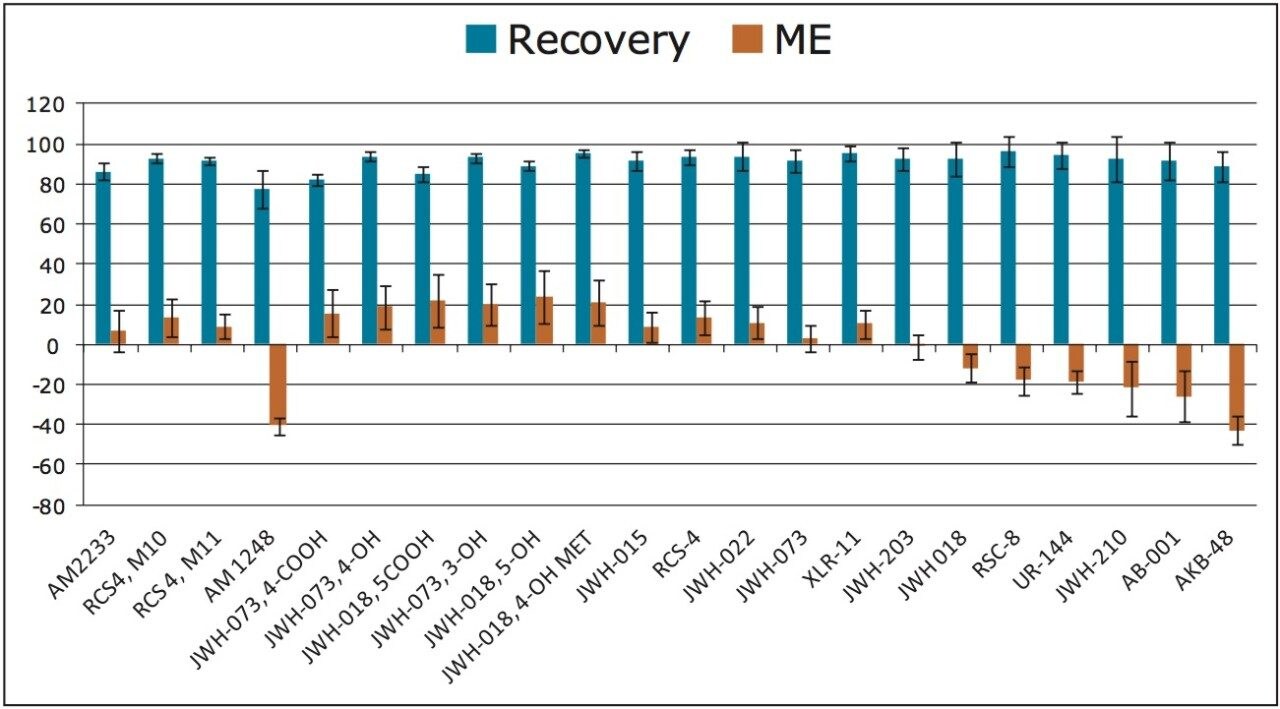
The synthetic cannabinoids and metabolites in this application include compounds that are neutral, acidic and basic. Use of the Oasis PRiME HLB Sorbent enabled the simultaneous extraction of all of the compounds and metabolites tested, regardless of their functionality. Recoveries and matrix effects (ME) were calculated according to the equations described in the experimental section and the results are shown in Figure 2. This extraction protocol results in nearly complete recovery for all compounds and minimizes matrix effects for the majority of analytes. All but one compound had recoveries of 80% or greater with an overall average recovery of 91%. Recoveries were consistent with an average %RSD at 5% across all compounds. Matrix effects across the panel were excellent. Only two compounds had matrix effects that slightly exceeded 40%, and all remaining compounds had matrix effects less than 25%. The average magnitude of matrix effects was only 17%. The high recoveries and minimal matrix effects for this panel of synthetic cannabinoids indicate that Oasis PRiME HLB should give similar results for other related compounds with a simple load-wash-elute protocol.
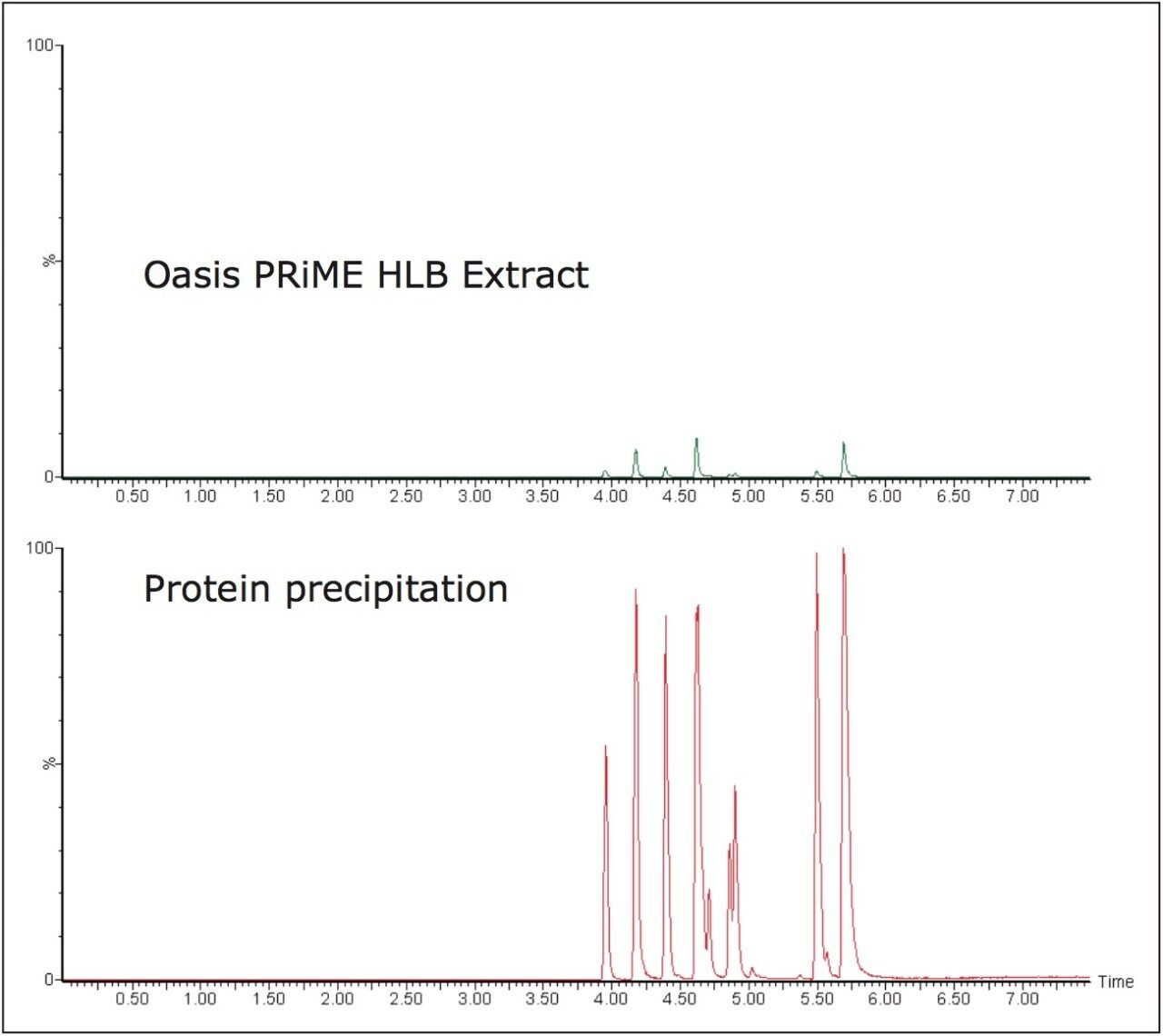
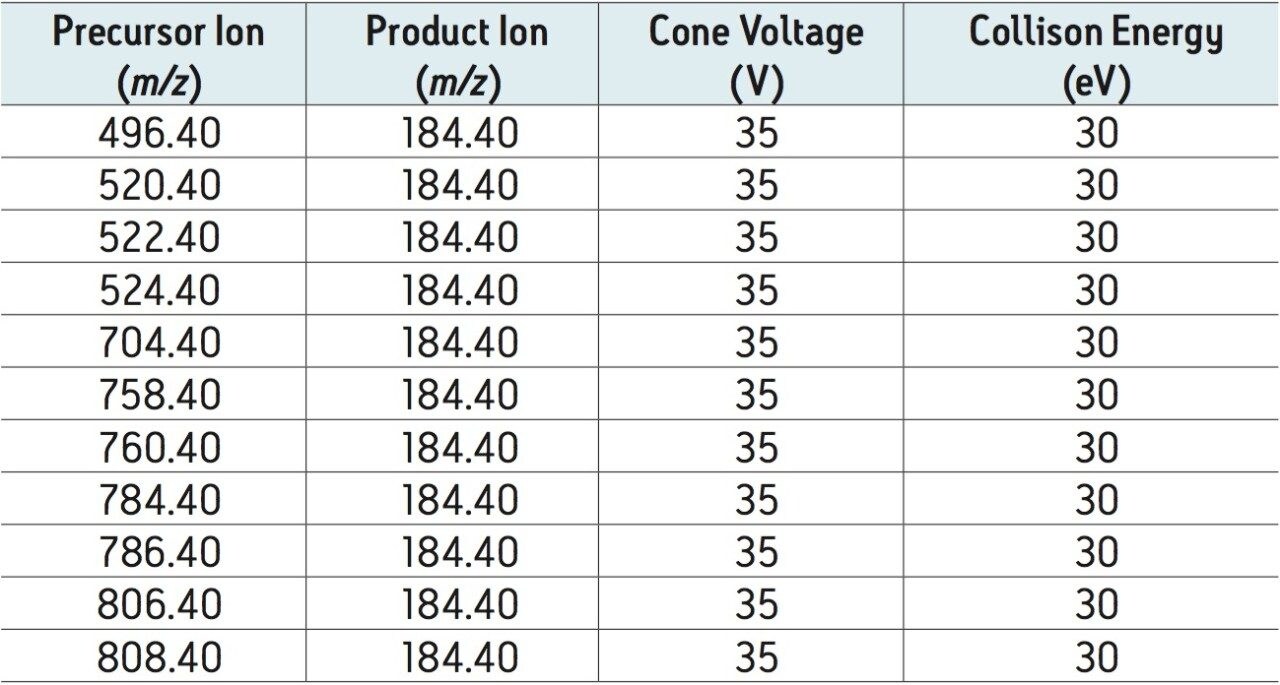
One of the key attributes of Oasis PRiME HLB, is its ability to deliver cleaner extracts than other sample preparation methods. One way that this is achieved is by removing endogenous phospholipids. Figure 3 shows chromatograms of combined phospholipid traces from an Oasis PRiME HLB extract and an identical sample subject to protein precipitation. Compared with protein precipitation (PPT), Oasis PRiME HLB removes over 95% phospholipids (Figure 3) resulting in a much cleaner extraction. This can translate to reduced matrix effects, longer column lifetimes, and less mass spectrometer source maintenance.
In order to assess linearity and analytical sensitivity, calibration curves were extracted at concentrations ranging from 0.2–100 ng/mL for all components. Quality control samples (N=4) at 2.5, 7.5, and 75 ng/mL were also extracted and analyzed. Table 2 summarizes R2 values from the calibration curves and QC summary data for all compounds. Quality control (QC) results were accurate and precise at low, medium and high concentrations. Accuracies for low level QC samples (2.5 ng/mL) ranged from 95–110% (except one compound, AM2233) with an average of 102%. The results for the medium and high QC levels were excellent for all analytes except one, with all accuracies within 15% of expected values. Analytical precision was excellent with most % RSDs less than 10% and none greater than 15%. When accuracy was assessed over all levels (low, medium, and high), the means ranged from 93% to 104%. Limits of quantification of 0.1 ng/mL were reached for most of the analytes and were no greater than 1 ng/mL. These results were achieved without the use of deuterated internal standards, once again demonstrating the consistency associated with Oasis PRiME HLB.
This application note highlights the use of Oasis PRiME HLB, a novel reversed-phase SPE sorbent which is designed to enable simple and fast SPE protocols while nearly eliminating endogenous phospholipids. Employing a simple load-wash-elute strategy, without any sorbent conditioning or equilibration, a panel of 22 synthetic cannabinoids was extracted from whole blood samples. Extraction recoveries averaged 91% across the entire panel, with an average matrix effect magnitude of only 17%. These results were consistent with mean %RSDs of 5% for all compounds. In addition, greater than 95% of phospholipids were removed vs. protein precipitation. Quantitative results were also excellent. Even without the use of deuterated internal standards, calibration curves were linear, with R2 values of 0.99 for 21/22 compounds. 97% of QC results were within 15% of target values and all %RSDs were less than 15%. In conclusion, Oasis PRiME has been used to achieve consistent, high recoveries with low matrix effects while virtually eliminating endogenous phospholipids from whole blood samples. The enabled excellent quantitiative results, even without the use of deuterated internal standards.
720005417, May 2015