Clarithromycin is manufactured globally by pharmaceutical companies, and is routinely analyzed in development and quality control laboratories. For this application, the separation of clarithromycin and clarithromycin related compound A is demonstrated across different column dimensions, using the USP compendial assay method for clarithromycin tablets.
Pharmaceutical drug manufacturers commonly use USP methods for routine analyses of formulated drug samples, and active pharmaceutical ingredients. Many of these methods were developed and validated on older column technology and instrumentation. Both column technology and chromatographic systems have significantly improved since these methods were developed. By using more modern columns on HPLC instrumentation, separations can be performed faster while meeting the specifications listed in the USP monograph. eXtended Performance (XP) 2.5-μm columns are designed to be used on both HPLC and UPLC instruments, allowing chromatographers to realize the benefits of smaller particle size columns and low-dispersion systems. The scalability of these columns also facilitates updating methods to comply with USP Chapter <621> guidelines. XP columns allow older methods to run faster on existing HPLC systems, and provide additional chromatographic and cost benefits when methods are transferred to UPLC systems.
Clarithromycin is a macrolide antibiotic that is used in the treatment of various bacterial infections, including tonsillitis, bronchitis, and pneumonia. The method being transferred in this application is the assay method for clarithromycin tablets,1 which is scaled from the original column on an HPLC system to XP columns on both HPLC and UPLC systems. XP columns are used to show their versatility when transferring methods adhering to USP guidelines. Updating the compendial method using XP columns can reduce analysis time, thereby, increasing sample throughput using HPLC instruments. The use of XP columns with UPLC instrumentation can provide improved sensitivity and resolution, as well as further solvent reduction over the course of the analysis, resulting in significant overall cost savings in a typical analytical laboratory.
Clarithromycin and clarithromycin related compound A USP standards were prepared in the mobile phase to a concentration of 0.5 mg/mL. The sample was placed in a TruView Maximum Recovery Vial for injection.
|
Mobile phase: |
65:35 methanol: 67 mM monobasic potassium phosphate, pH 4.0 with phosphoric acid |
|
Separation mode: |
Isocratic |
|
Detection: |
UV at 210 nm |
|
Columns (L1): |
XSelect CSH C18 4.6 x 150 mm, 5 μm; XSelect CSH C18 XP 4.6 x 75 mm, 2.5 μm; XSelect CSH C18 XP 4.6 x 50 mm, 2.5 μm |
|
Column temp.: |
50 °C |
|
Needle wash: |
95:5 ACN/water |
|
Sample purge: |
95:5 water/ACN |
|
Seal wash: |
50:50 MeOH/water |
|
Flow rate: |
Scaled with method |
|
Injection volume: |
Scaled with method |
|
Mobile phase: |
65:35 methanol: 67 mM monobasic potassium phosphate, pH 4.0 with phosphoric acid |
|
Separation mode: |
Isocratic |
|
Detection: |
UV at 210 nm |
|
Columns (L1): |
XSelect CSH C18 XP 4.6 x 75 mm, 2.5 μm; XSelect CSH C18 XP 4.6 x 50 mm, 2.5 μm; XSelect CSH C18 XP 2.1 x 75 mm, 2.5 μm |
|
Column temp.: |
50 °C |
|
Needle wash: |
95:5 ACN/water |
|
Sample purge: |
95:5 water/ACN |
|
Seal wash: |
50:50 MeOH/water |
|
Flow rate: |
Scaled with method |
|
Injection volume: |
Scaled with method |
|
Data management: |
Empower 3 Software |
|
USP resolution: |
Not less than (NLT) 2.0 between clarithromycin and clarithromycin related compound A |
|
USP plate count: |
NLT 750 from the clarithromycin peak |
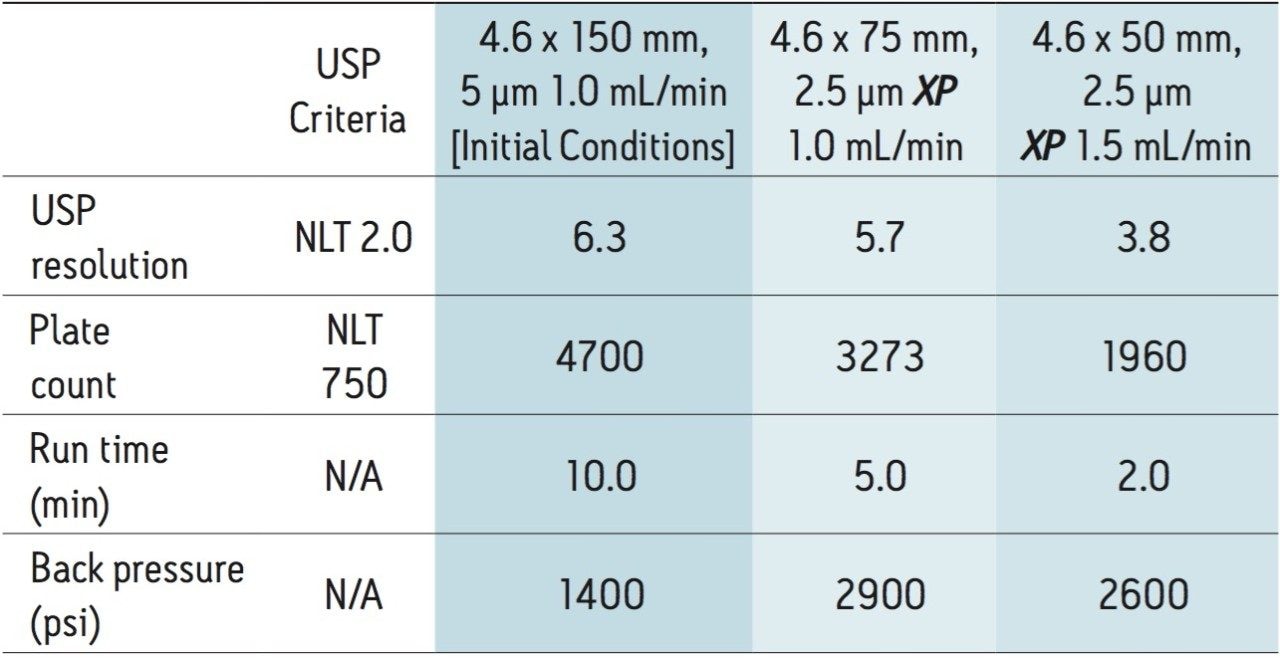
Clarithromycin is manufactured globally by pharmaceutical companies, and is routinely analyzed in development and quality control laboratories. For this application, the separation of clarithromycin and clarithromycin related compound A is demonstrated across different column dimensions, using the USP compendial assay method for clarithromycin tablets. Many USP methods use HPLC instrumentation with a 5-μm particle column, resulting in long analysis times and excessive solvent consumption. However, by using smaller particle size XP 2.5-μm columns, run times can be shortened, while maintaining the requirements specified in the assay. As a result of shorter analysis times, throughput can increase with less solvent used per analysis, resulting in overall operating cost savings and faster release of the product. The current USP <621> Chromatography chapter provides allowable method changes that include ±70% changes to column length, -50% in particle size, and ±50% flow rate. These guidelines were followed throughout the method transfers demonstrated here.
The original compendial assay method for clarithromycin requires the use of an L1 designated column. The USP-listed column for this assay is a Delta-Pak HPI C18. Using the Waters Reversed-Phase Column Selectivity Chart, a similar but more modern L1 designated XSelect CSH C18 Column was chosen. The XSelect CSH C18 Column provides scalability between the HPLC and UPLC systems, with the availability of smaller particle sizes for faster analysis. The USP method for clarithromycin was first run using the original compendial method conditions on an Alliance HPLC System with a 4.6 x 150 mm, 5 μm column at a flow rate of 1.0 mL/min. All of the assay suitability requirements were well within specified USP criteria, as shown in Table 1.
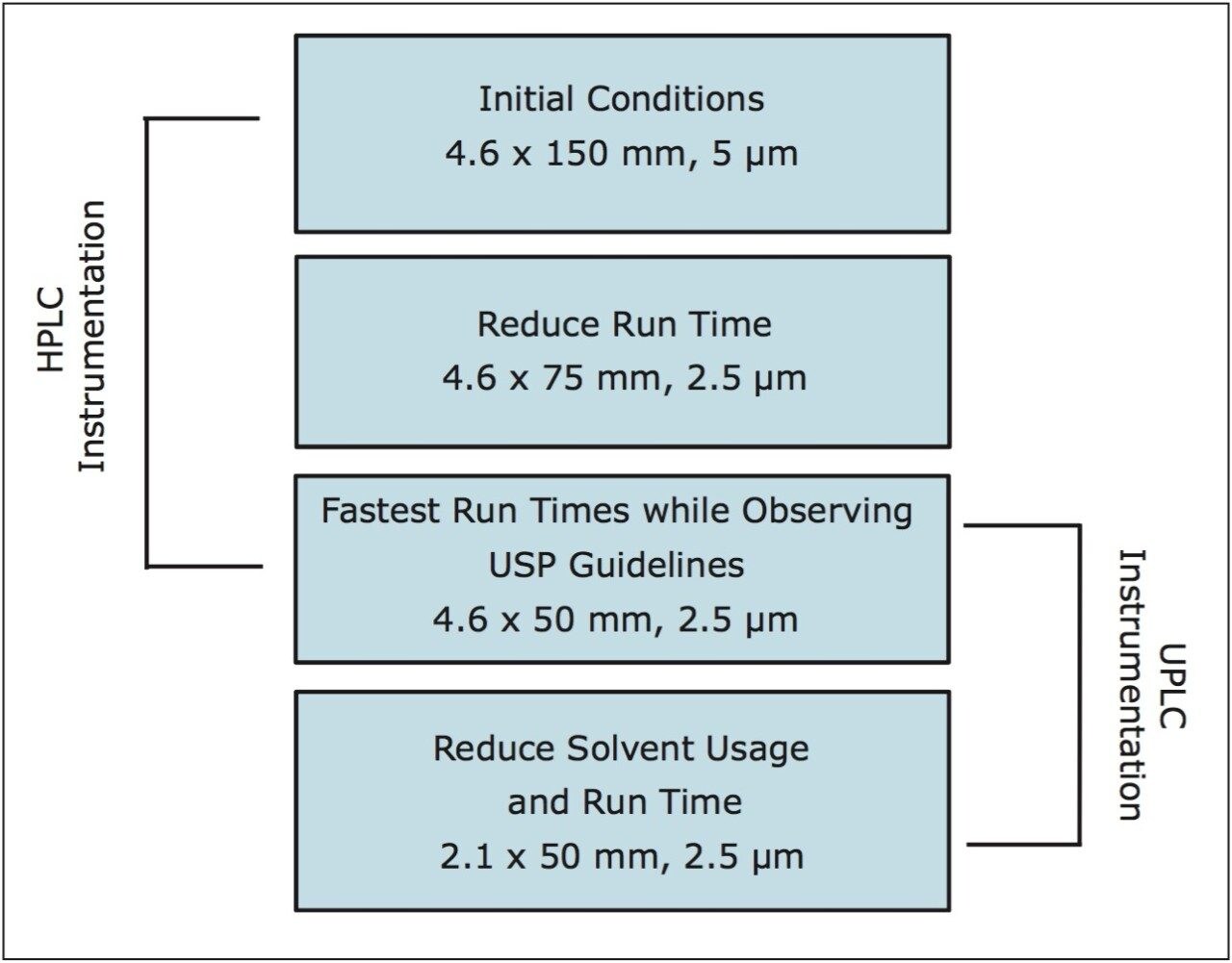
The versatility of scaling the compendial method using XP 2.5-μm columns across different systems, while remaining within USP <621> guidelines, is shown in Figure 1. eXtended Performance [XP] columns are 2.5-μm HPLC and UPLC columns packed to a high efficiency, and designed to withstand the higher pressures of a UHPLC system, allowing the XP columns to be run on both HPLC and UPLC instruments.
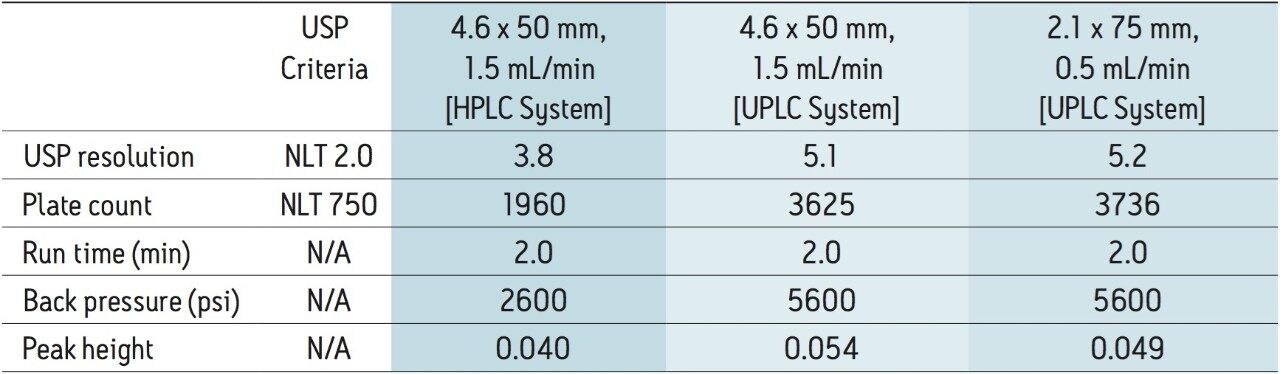
The compendial method was first transferred to an XP 4.6 x 75 mm 2.5 μm column using the ACQUITY UPLC Columns Calculator.2 The length to particle size ratio (L/dp) of the original column (30,000) was maintained to ensure that the resolving power of each column was comparable. Proper transfer of the original method requires an increase in flow rate to 2.0 mL/min, which is outside the allowable changes in USP <621> guidelines. In this case, the flow rate was kept at the original condition requirement of 1.0 mL/min, due to back pressure limitations of the HPLC system. The original separation of clarithromycin and clarithromycin related compound A, along with the comparable separation on the XP 4.6 x 75 mm, 2.5 μm column, are shown in Figures 2A and 2B, respectively. The XP 4.6 x 75 mm column shows a 50% reduction in run time, while maintaining the resolution criteria specified in the USP method. Because the method was not able to be properly scaled for flow rate, there is a modest reduction in plate count and resolution, as shown in Table 1, but the results are still well within assay suitability criteria of NLT 2.0.
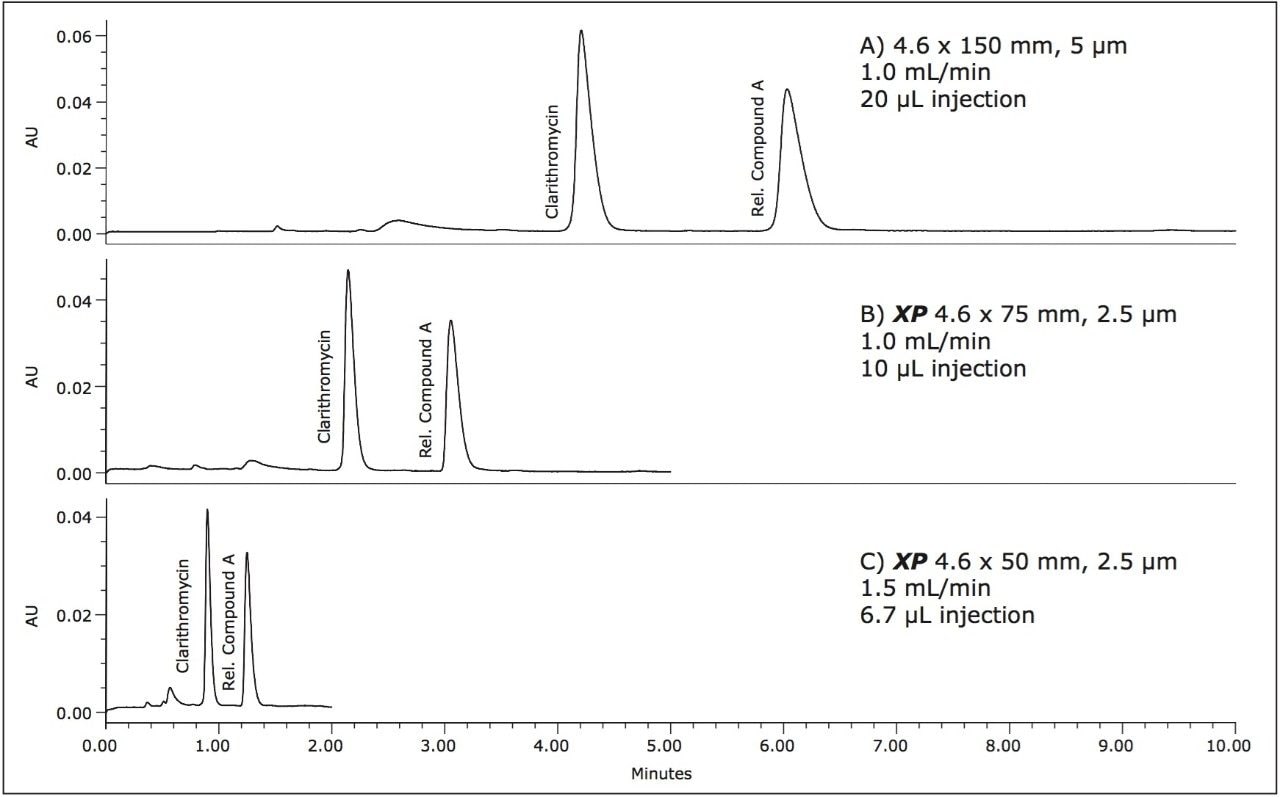
Since assay methods do not always necessitate the stringent resolution that impurities or other methods may require, a shorter XP 4.6 x 50 mm, 2.5 μm column was also tested to demonstrate the fastest analysis and sample throughput possible using HPLC under allowable USP <621> Chromatography guidelines. The separation on the XP 4.6 x 50 mm, 2.5 μm column was performed at 1.5 mL/min (fastest allowable flow rate within USP guidelines). Using the XP 4.6 x 50 mm, 2.5 μm column with a flow rate of 1.5 mL/min, an 80% reduction in analysis time can be achieved, as shown in Figure 2C. Since the 4.6 x 50 mm column has a lower resolving power (L/dp 20,000) compared to the original column, the resolution for the separation is reduced to 3.8; however, both plate count and resolution requirements remain well within assay specifications, as seen in Table 1. By using XP 2.5-μm columns on HPLC instrumentation, the assay for clarithromycin is performed up to 80% faster, and uses up to 70% less solvent per run than the original compendial method column, while still meeting USP assay suitability criteria.
As described in the method transfer flowchart in Figure 1, the assay was next transferred from the Alliance HPLC System to an ACQUITY UPLC H-Class System. Newer instrumentation, such as the ACQUITY UPLC H-Class System, can result in faster, more efficient separations due to high back pressure capabilities, faster equilibration between injections, and significantly lower system volume and dispersion. To compare the separation capabilities between the HPLC and UPLC systems, the assay method using the XP 4.6 x 50 mm, 2.5 μm column, shown in Figure 2C, was re-run on an ACQUITY UPLC H-Class System, shown in Figure 3B. Using the same method and XP column, the change in instrumentation alone from HPLC to UPLC resulted in a 25% increase in resolution and a 26% increase in sensitivity based on peak height, as shown in Table 2.
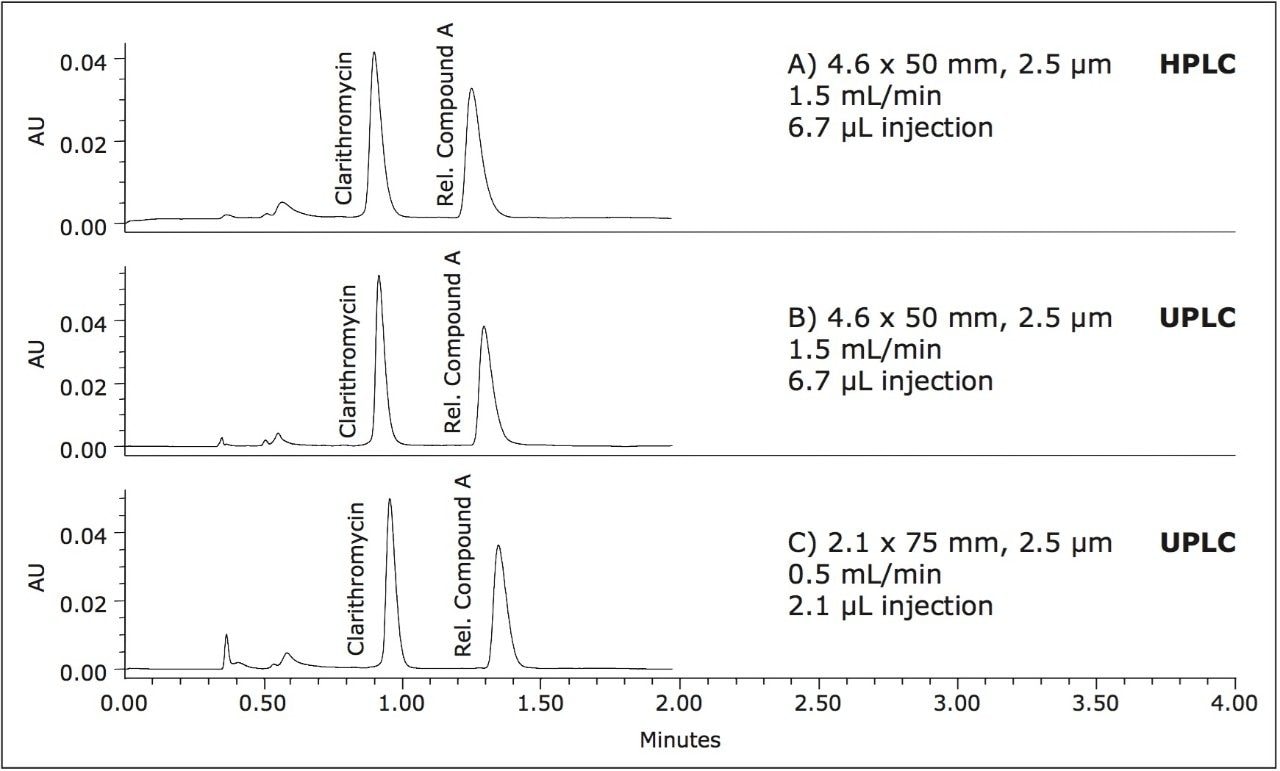
Finally, an XP 2.1 x 75 mm, 2.5 μm column was tested to demonstrate that, by reducing the column interior diameter (I.D.) and maintaining the L/dp of 30,000, acceptable resolution can be obtained while reducing solvent usage even further. The proper flow rate for this separation, according to the ACQUITY UPLC Columns Calculator, was 0.42 mL/min; however, this flow rate is outside of the USP <621> guidelines for allowable changes to the flow rate. Consequently, a flow rate of 0.5 mL/min was used to maintain compliance. The resulting chromatogram for this separation, shown in Figure 3C, demonstrates an 80% reduction in run time compared to the original HPLC compendial method conditions, shown in Figure 2A, and assay suitability requirements are still easily met, as listed in Table 2. Furthermore, by using an XP 2.1 x 75 mm column, the solvent use per analysis is reduced by 90% compared to the original compendial method column, and 30% less than the fastest XP 4.6 x 50 mm method, resulting in significant cost savings.
Using eXtended Performance [XP] 2.5 μm columns on existing HPLC systems, run times and solvent usage can be reduced by up to 80% and 70%, respectively, compared to the original compendial USP procedure. By combining XP columns with UPLC instrumentation, run times can be reduced by up to 80%, while reducing solvent usage by 90%. The availability of XP columns, which are capable of being run on both HPLC and UPLC instruments, allow USP methods to be updated, while following the current <621> guidelines. By using XP columns on HPLC instrumentation, older methods can be performed faster. When switching to UPLC systems, additional chromatographic and cost benefits can be obtained. In any typical analytical laboratory, modernizing USP methods using columns with smaller particle sizes can result in significant savings of time and costs.
720004461, October 2012