This is an Application Brief and does not contain a detailed Experimental section.
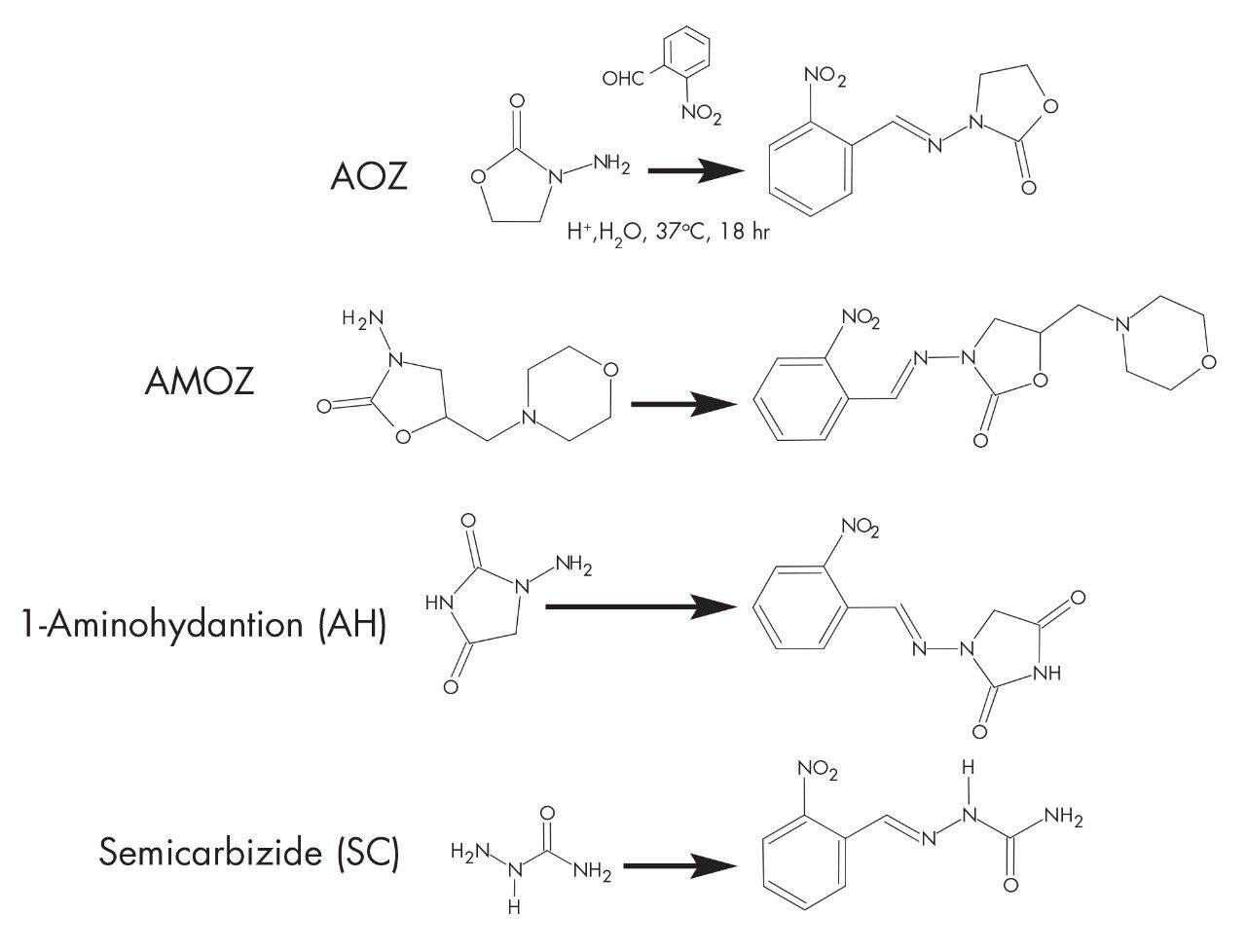
This application describes the methods to determine Nitrofurans in food producing animal tissues.
The United States Food and Drug Administration (US FDA) banned Nitrofuran drugs are banned in food producing animals because they pose a public health risk. The rule went into effect as a result of evidence that the drugs may induce carcinogenic residues in animal tissues.
1. Homogenize 10 g of sample in 100 mL of 0.12 M hydrochloric acid.
2. Take 1 mL aliquot and treat with 400 μL of 50 mM 2-nitrobenzaldehyde in dimethylsulfoxide.
3. Hydrolyze/derivatize the sample for 16 hours at 37 °C.
4. Adjust the sample to pH 7.4 with potassium hydrogen phosphate.
5. Centrifuge sample for 5 minutes at 8000 rpm
|
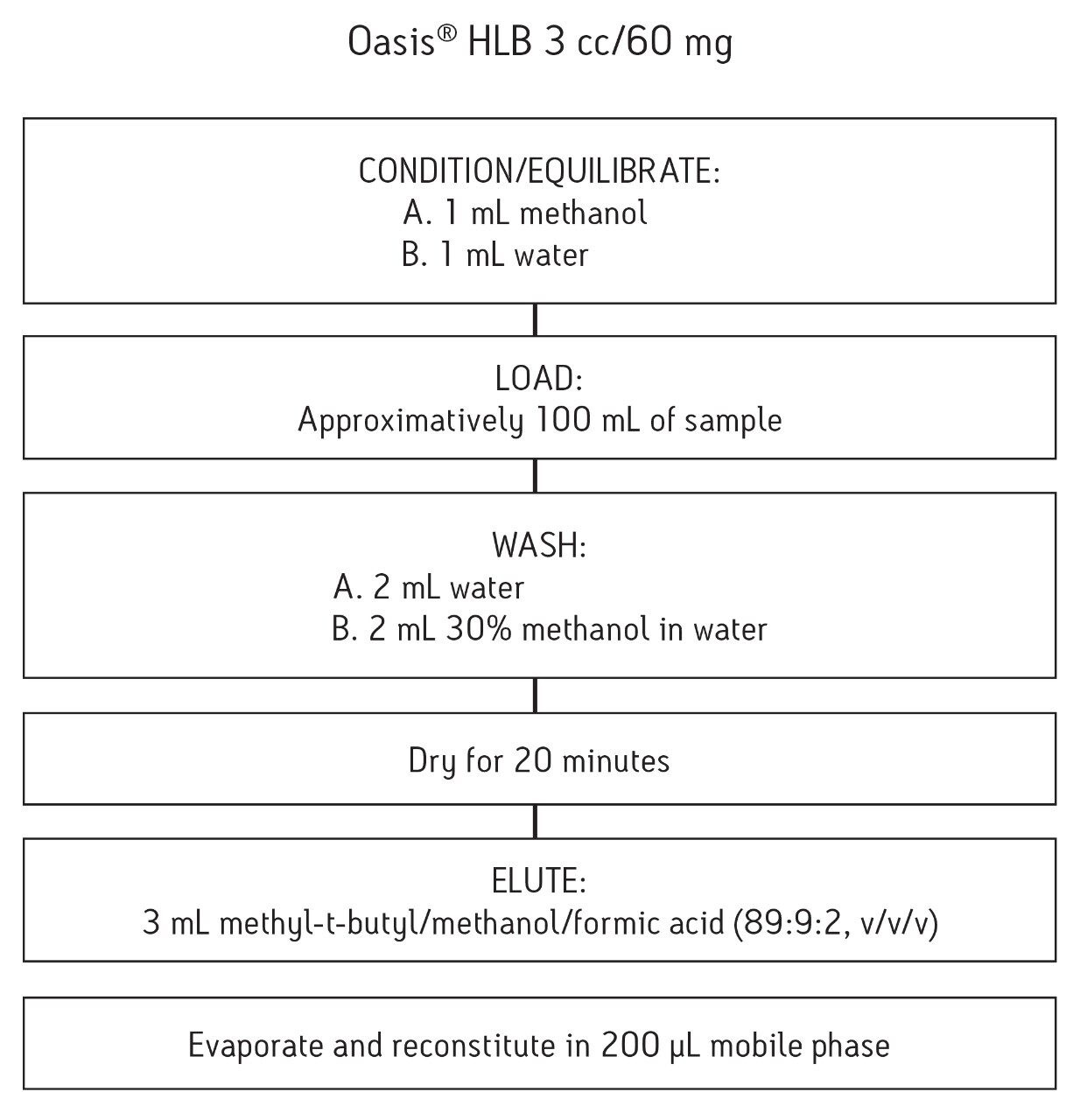
System: |
Alliance HPLC 2695 |
|
Column: |
XTerra MS C18, 3.5 μm, 2.1 x 100 mm |
|
Flow rate: |
0.2 mL/min |
|
Mobile phase: |
Isocratic 70% 20 mM ammonium formate pH 4, 30% acetonitrile |
|
Injection volume: |
20 μL |
|
Column temp.: |
30 °C |
|
MS System: |
Waters Quattro micro API |
|
Ionization mode: |
Positive electrospray (ESI+) Multiple reaction monitoring |
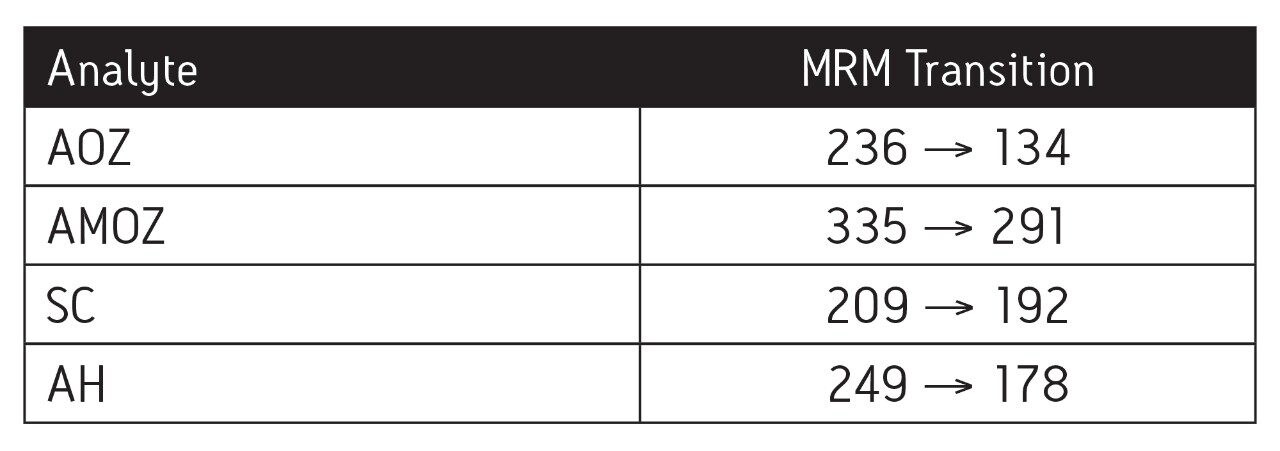
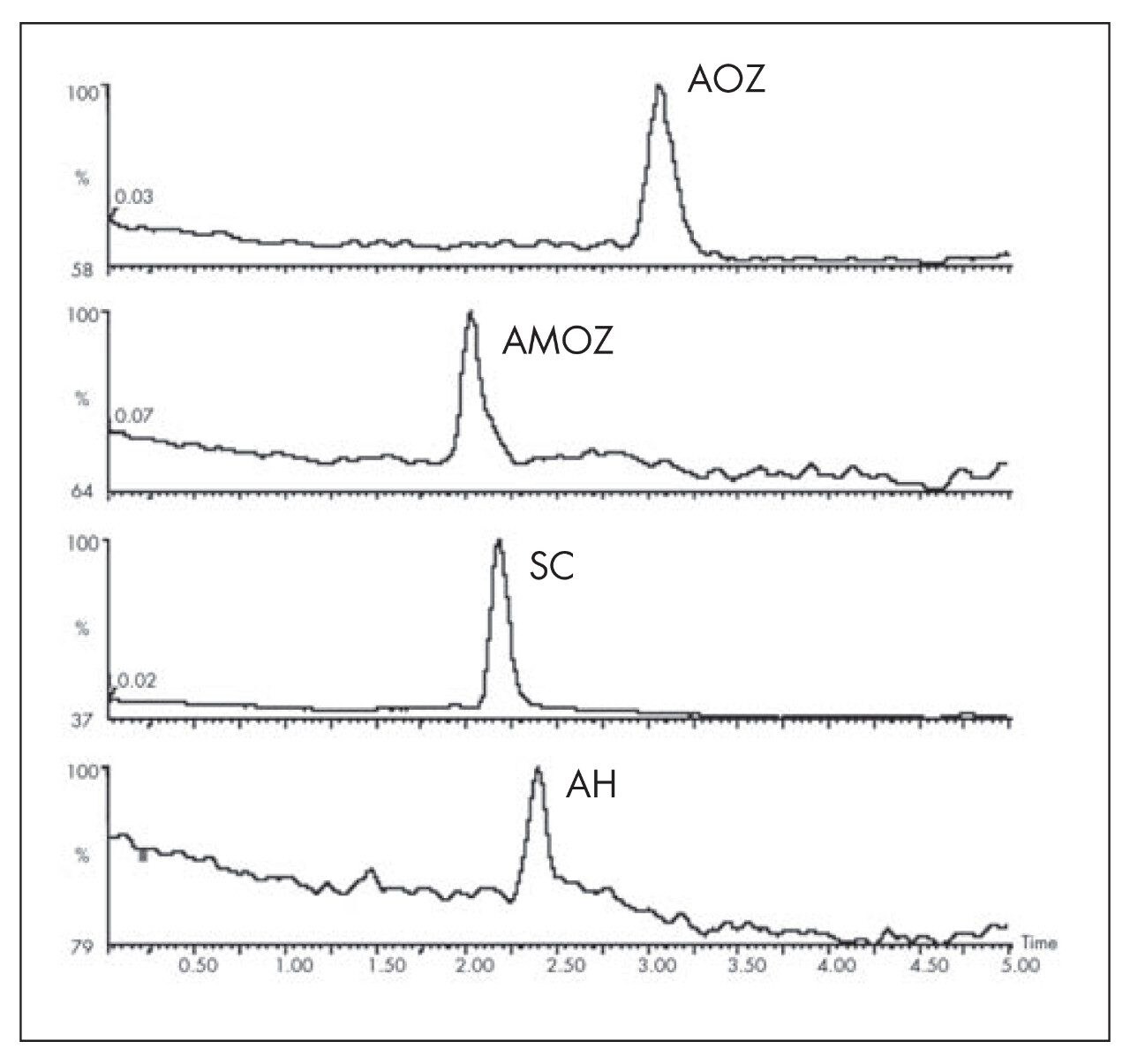
Spiked chicken muscle (1 ng/g) metabolites as 2-nitrobenzaldehyde derivatives.
720002594, April 2008