Increased Identification Confidence for Extractables Screening Using the Xevo™ MRT Mass Spectrometer
Rachel Sanig, Jayne Kirk, Lee Gethings, Richard Lock
Waters Corporation, United States
Published on September 10, 2025
Abstract
Due to concerns about the safety of components from plastic, it is crucial to screen for potential extractables in pharmaceutical packaging and medical devices. Compounds found at levels above the analytical evaluation threshold must be identified and reported for toxicological assessment. Achieving highly confident compound identifications can be challenging due to the complexity of samples, potential false positives, and the multiple compound identifications for each chromatographic peak. Here, an extractables analysis using a benchtop multi-reflecting time-of-flight (MRT) mass spectrometer, the Xevo MRT Mass Spectrometer, is reported, operating in a data independent acquisition (DIA) mode. High mass accuracy for precursor and fragment ion data is attained which is critical to ensure high identification confidence for a screening workflow.
Benefits
- The Xevo MRT Mass Spectrometer provides consistent low- to sub-ppm mass accuracy, allowing for increased extractables identification confidence.
- High mass resolution, sensitivity, and fast acquisition rates ensure exceptional data quality for scientific interpretation, suited to large complex data sets.
- A DIA workflow delivers accurate mass of both precursor and associated fragment ion data further increasing the confidence of identifying unknowns when screening against a library and aiding structural elucidation.
- The UNIFI™ application within the waters_connect™ Software provides customized workflows to simplify screening and structural elucidation in complex datasets.
Introduction
Medical devices, pharmaceutical packaging, and manufacturing components contain different chemicals, including polymers, polymer additives such as antioxidants, slip agents, colorants, and other compounds. These chemicals, their impurities, and degradation products can migrate out of the materials resulting in potentially unsafe substances. Due to this, there are regulations, standards, and guidance in place to ensure that safety limits for the consumer are met.1-3
To ensure safety, it is therefore crucial to screen for and identify potential extractables and leachables (E&L). Compounds that are found at levels above the analytical evaluation threshold (AET) must be identified and reported for toxicological assessment.4 The confidence level in the identifications of these compounds must be as high as possible, which can be challenging due to the complexity of samples, potential false positives, and the number of possible compound identifications for each chromatographic peak. Technological advancements are critical to assist in reducing the burden of this process on the analyst.
The Xevo MRT Mass Spectrometer (Figure 1) utilizes a novel quadrupole-multi reflecting time-of-flight design that delivers mass resolution of up to 100,000 full width half maximum (FWHM) at 50 Hz (MS) and 100 Hz (MS/MS) scan rates, resulting in low- to sub-ppm mass accuracy across a wide m/z range for confident extractables screening analyses. The system can be employed using the DIA approach, MSE,5 that ensures the acquisition of precursors and their associated fragment ions with low- to sub-ppm mass accuracy. Combining the DIA strategy with the Xevo MRT Mass Spectrometer attributes significantly reduces the possibility of false positives, while greatly reducing the number of potential candidate compounds per chromatographic peak, hence increasing identification confidence. This data is also critical for confident structural elucidation of unknowns that is necessary for an extractables screening report.
Here, an extractables analysis is reported using the Xevo MRT Mass Spectrometer with a data independent acquisition workflow. The subsequent data is processed using the compliance ready waters_connect Software with the UNIFI application that provides automated data acquisition, processing, and reporting.

Experimental
Sample Description
Three different commercially available antihistamine tablet products were purchased and three cuttings from each plastic and foil packaging were taken. Each cutting was thermally shaken at 50 °C in isopropanol (IPA) for 24 hours alongside a procedural solvent blank (negative control) and a pooled quality control (QC). Samples were spiked with the Waters Extractables and Leachables Screening Standard (E&L SST) (p/n: 186006063) prior to injecting as technical triplicates.
Method Conditions
LC Conditions
|
LC system: |
ACQUITY Premier System |
|
Column: |
ACQUITY CORTECS™ C18, 90 Å, (1.6 μm, 2.1 x 100 mm) |
|
Column temperature: |
50 °C |
|
Injection volume: |
1 µL |
|
Flow rate: |
0.3 mL/min |
|
Mobile phase A: |
Water + 1 mM ammonium acetate + 0.1% formic acid |
|
Mobile phase B: |
Methanol |
|
Gradient: |
Mobile phase B was held at 2% for 0.5 minutes before being ramped to 98% over 5.5 minutes then held for 7 minutes. It then dropped to 2% for 2 minutes. |
MS Conditions
|
MS system: |
Xevo MRT Mass Spectrometer |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
m/z 50–1200 |
|
Source temperature: |
120 °C |
|
Desolvation temperature: |
550 °C |
|
Desolvation gas flow: |
800 L/hr |
|
Cone gas flow: |
50 L/hr |
|
Capillary voltage: |
2.5 kV |
|
Collision energy: |
Low energy: 6 eV High energy ramp: 20–40 eV |
|
Cone voltage: |
40 V |
Data Management
The waters_connect Software was used for data acquisition and the UNIFI application was used for data processing. All statistical analyses were undertaken with EZInfo® 3.0 (Sartorius, Göttingen).
Results and Discussion
A complete extractables analysis using an E&L specific workflow can be undertaken with the UNIFI application (Figure 2A). The workflow can be customized to meet user requirements and help streamline the analysis of complex datasets.
System Suitability Review
The first step undertaken in the workflow is to review the E&L SST mix results, used to benchmark the system (Figure 2). The E&L SST contains common polymer additives over a range of molecular weights and ionization polarities. The Xevo MRT Mass Spectrometer delivers consistent low- to sub-ppm mass accuracy with mass accuracy for all E&L SST analytes across all injections providing an RMS of 0.81 ppm.
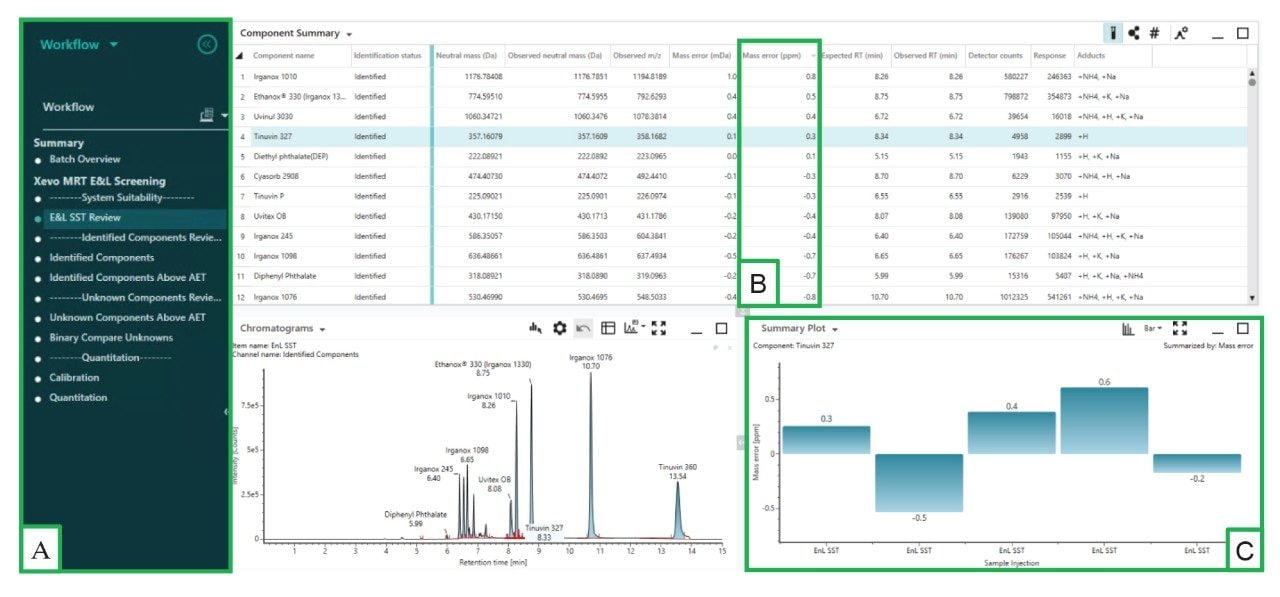
During data processing, precursor and fragment ions resulting from the MSE acquisitions are precisely aligned based on their retention times. The combined high mass accuracy of precursors and associated fragment ions for all compounds (Figure 3)6 provides comprehensive and confident extractables screening results.
![[A] Low (upper) and high (lower) energy spectra of diethyl phthalate](/content/dam/waters/en/app-notes/2025/720008970/720008970en-f3.jpg.82.resize/img.jpg)
Screening Against an E&L Library
When undertaking an extractables analysis, any compounds that are found above the AET need to be identified and reported for toxicological assessment. The AET is defined as the level below which identification and quantification is not required.4 After verifying the SST mix, the samples were investigated by screening against the Waters E&L library to find matches based on accurate mass, retention time, and corresponding fragments.7
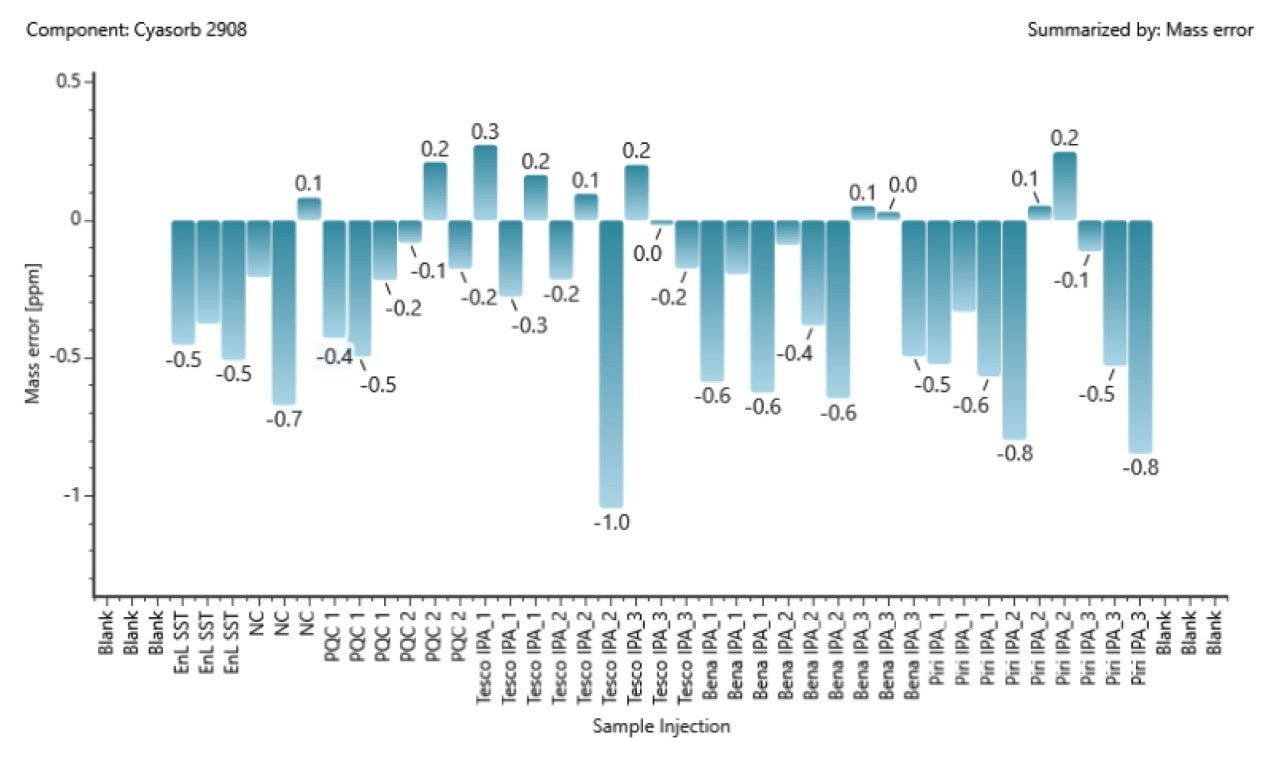
With these parameters available in a library or database for each compound entry, the possibility of false positives is reduced, the number of possible candidates is reduced, and confidence in any identification made is increased. With consistent low- to sub-ppm mass accuracy this confidence is further increased. To ensure the mass accuracy is maintained across the study, the E&L SST was spiked into all samples (Figure 4) and monitored, with fragment ion mass accuracy also confirmed to be low- to sub-ppm (Figure 5). The UNIFI application utilizes both experimental fragment ion data and theoretical fragments with in-silico fragment matching.
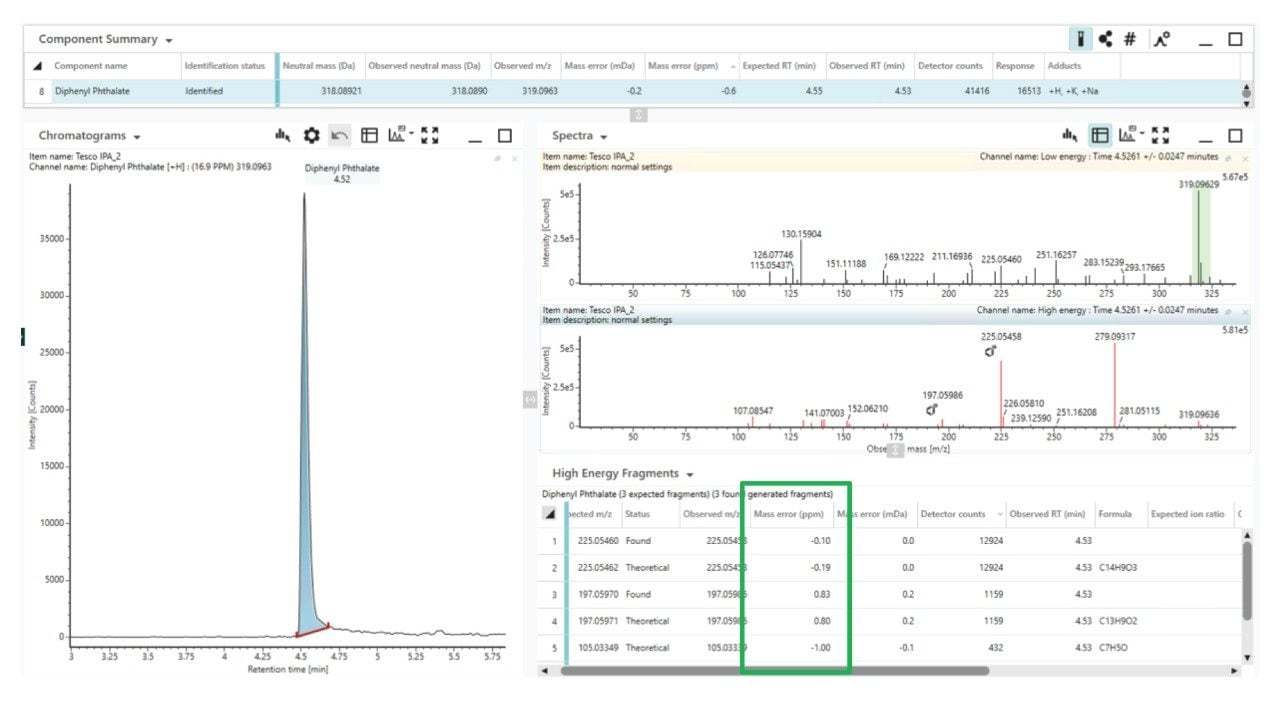
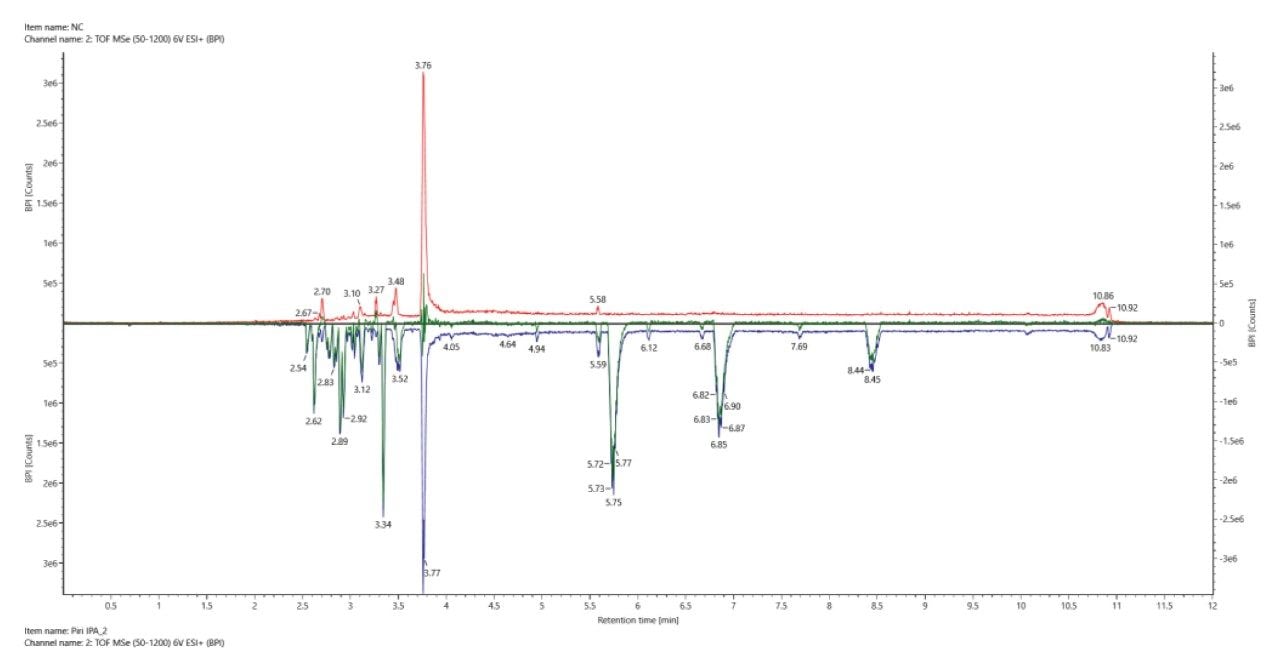
To assist with the review of potential identifications, the UNIFI application has a number of tools to help streamline this process. The Binary Compare Tool is used to compare the samples to the extracted procedural blank (negative control) to find components that are unique to the sample or elevated in the sample compared to the blank (Figure 6).
Once the binary compare tool is applied, this can be incorporated as a data filter. Filters can be applied to significantly reduce the number of potential candidates aiding the data review process and reducing the number of false positives. Different parameters can be applied to showcase differing confidence levels. For example, the number of matched fragments, a certain response level to apply an AET threshold, unique or elevated response compared to the negative control, and limiting the mass error range (Figure 7).
![[A] Example of filters that can be applied within the UNIFI application](/content/dam/waters/en/app-notes/2025/720008970/720008970en-f7.jpg.82.resize/img.jpg)
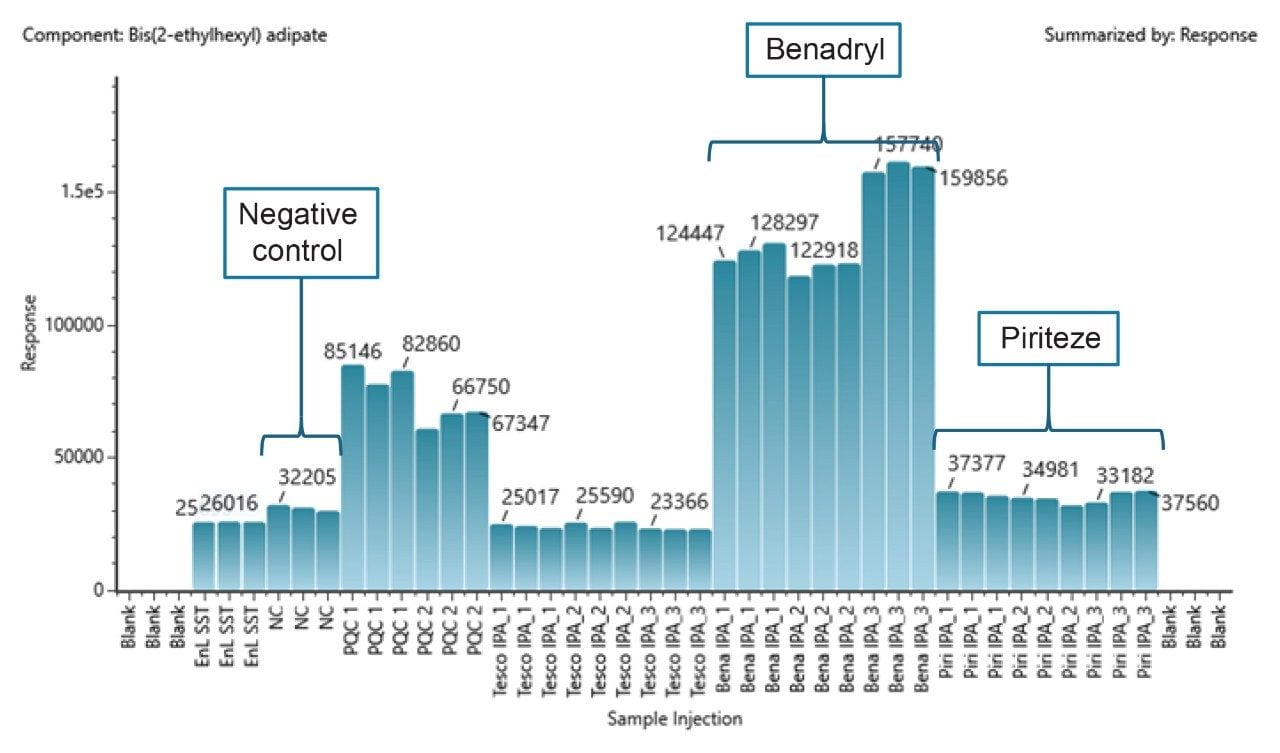
The trend plot view within the UNIFI application allows the response profiles for the identified compounds across a sample batch to be viewed. For example, bis(2-ethylhexyl)adipate was found, using the Waters E&L library, across all injections (mass error (RMS)=0.77 ppm) but elevated in two of the packaging types analyzed (Figure 8).
Statistical Analysis and Structural Elucidation
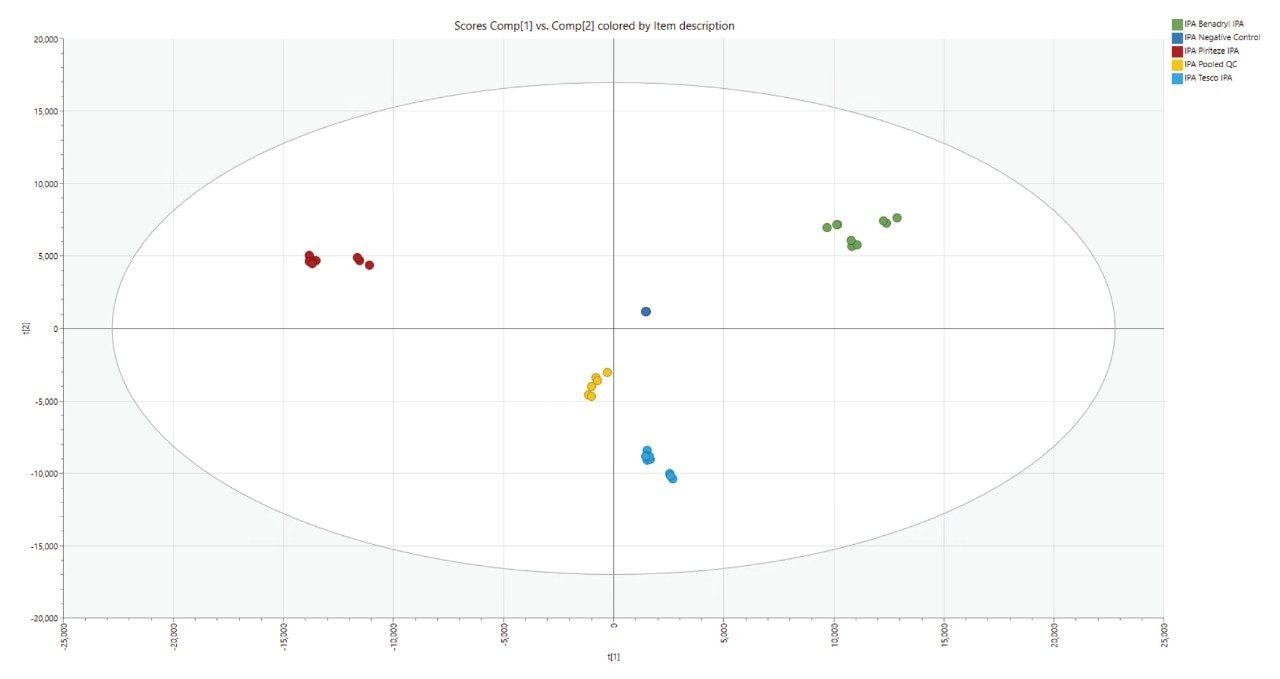
Multivariate statistical analysis (MVA) tools can be used for the analysis of complex datasets. Data were transferred to EZInfo using the UNIFI application, and Principal Component Analysis (PCA) was performed. Figure 9 shows the resulting PCA scores plot from the negative control, the three samples (including biological and technical replicates), and the pooled QC. There is clear separation between the three groups of samples, which highlights the difference between the extraction profiles of these packaging materials. Tight clustering of the pooled QC injections signifies the high technical reproducibility achieved with the Xevo MRT Mass Spectrometer.
To establish the features responsible for the differences between sample groups, an Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) was performed (Figure 10A), highlighting the variation between groups. The inter group variation of the sample is expected as the samples were not milled down to promote homogeneity.
The resulting S-Plot indicates the differential markers between the two groups compared from the OPLS-DA (Figure 10B). These markers were selected and transferred back into the UNIFI application for structural elucidation.
![OPLS-Da [A] and S-Plot [B] representing the negative control and one of the sample types](/content/dam/waters/en/app-notes/2025/720008970/720008970en-f10.jpg.82.resize/img.jpg)
The imported differential markers are then displayed using the Discovery Tool in the UNIFI application.8 The trend plot for one of the elevated markers is displayed (Figure 11A). Since MSE mode was used, the accurate mass of both precursor and fragments ions were available for the interpretation of each marker.
The Discovery Tool combines elemental composition determination with database searching and in silico fragmentation. The elemental composition calculator determines the most likely chemical formulae for a precursor ion. The i-FIT algorithm is used to rank formulae by the likelihood that the theoretical isotope pattern of the formula matches the isotope pattern in the measured spectrum. Each elemental composition that explains a given precursor ion is automatically searched against the ChemSpider database (Royal Society of Chemistry), returning prospective compounds, together with their structures, for a given composition. The structure of each compound is then automatically submitted to an in-silico fragmentation algorithm and the m/z values of the theoretical fragments are compared to the high energy m/z values of the marker. Figure 11B demonstrates the information displayed by the Discovery Tool for an elucidated marker. A standard would be needed for a 100% match, but this greatly reduces the processing time for the analyst.
![MVA marker results and Discovery Tool [A] Marker summary across all injections for one marker](/content/dam/waters/en/app-notes/2025/720008970/720008970en-f11.jpg.82.resize/img.jpg)
Conclusion
Screening with the Xevo MRT Mass Spectrometer is a highly specific tool for E&L screening analyses. The multi reflecting-time of flight technology ensures consistent low- to sub-ppm mass accuracy for the wide mass range typical of extractable compounds. For example, the mass accuracy for all E&L SST analytes across standard injections had an RMS of 0.81 ppm.
Utilizing MSE mode provides highly informative and accurate data for both precursor and fragment ions in sample matrix. This reduces the false positive rate and significantly reduces the possible candidate matches therefore greatly increasing identification confidence. For example, from no filters to applying fragment matches, binary compare, and mass accuracy filters of ±5 ppm, reduced the number of candidate matches by 80%. Narrowing the mass accuracy window to ±1 ppm further reduces the matches by another 60% in this case, reducing the burden on the analyst.
MSE mode is also critical to assist with the structural elucidation of unknowns, for example, a putative identification of N,N’-1,12-dodecanediyldidodecanamide was made with a mass error –0.77 ppm and multiple fragment matches.
Software tools were employed to aid review of the highly accurate mass data within complex datasets. These included binary compare, filtering, multivariate statistical analysis, and trend plots. This combined with the technology of the Xevo MRT Mass Spectrometer, ensures a seamless and highly confident extractables screening workflow.
References
- USP-NF/PF. <1664> Assessment of Drug Product Leachables Associated with Pharmaceutical Packaging/Delivery Systems. https://doi.usp.org/USPNF/USPNF_M7127_03_01.html
- USP-NF/PF. <1663> Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems. https://doi.usp.org/USPNF/PNF_M7126_03_01.html
- Norwood D., Paskiet D., Ruberto M., Feinberg T., Schroeder A., Poochikian G., Wang Q., Deng T., Degrazio F., Munos M., Nagao L. Best Practices for Extractables and Leachables in Orally Inhaled and Nasal Drug Products: An Overview of the PQRI Recommendations. Pharmaceutical Research. 25. 727–39, 2008.
- ISO 10993-18:2020 Biological evaluation of medical devices — Part 18: Chemical Characterization of Medical Device Materials Within a Risk Management Process, https://www.iso.org/standard/64750.html
- Plumb, R S et al. “UPLC/MS(E); A New Approach for Generating Molecular Fragment Information for Biomarker Structure Elucidation.” Rapid communications in mass spectrometry : RCM vol. 20,13 (2006): 1989-94.
- Stevens D., Cabovska B., Bailey A. Detection and Identification of Extractable Compounds from Polymers. Waters Application Note. 720004211. January 2012.
- McCullagh M., Mortishire-Smith R. J., Goshawk J., Cooper J., Obkircher M., Sprecher H., Koehling R., Nold M., Jacobsen J., Sanig R. Extractables, Leachables, and Contact Materials: The Invaluable Benefit of Ion Mobility-Enhanced Mass Spectrometry Libraries. Waters Application Note. 720007655. June 2022.
- Cabovska B. Screening Workflow for Extractable Testing Using the UNIFI Scientific Information System. Waters Application Note. 720005688. April 2016.
Featured Products
720008970, August 2025