This is an Application Brief and does not contain a detailed Experimental section.
This Application brief demonstrates the use of an ACQUITY QDa Mass Detector integrated with an ACQUITY Diverter Valve for the analysis of aryl esters of p-toluenesulfonic acid in monitoring of potential genotoxic impurities.
Minimize potential sources of contamination and mitigate chances of ion suppression by diverting selected analysis segments to waste.
Genotoxic impurities (GTIs) have the potential to react with DNA and induce genetic mutation, which may consequently lead to cancer. It is therefore essential to have a reliable and highly sensitive method for the low level detection of these mutagenic impurities in both drug substance and drug product assays.
For low levels analysis, it is important to show that impurities can be separated and accurately measured in the presence of the sample matrix. The sample matrix can often enhance or suppress the MS signal, leading to inconsistencies in quantitation from sample-to-sample. In addition, the presence of a high concentration of active pharmaceutical ingredient (API) may contaminate the MS source, generating inaccurate results. Incorporating a diverter valve into the flow path will enable the matrix components to be selectively directed to waste, avoiding contamination of the MS source and ensuring the consistency and accuracy of the response.
In this study, we will demonstrate the use of an ACQUITY QDa Mass Detector integrated with an ACQUITY Diverter Valve for the analysis of the potentially genotoxic impurities, methyl and ethyl esters of p-toluenesulfonic acid.
The ACQUITY Diverter Valve connected to an ACQUITY QDa Mass Detector enables users to switch the mobile phase flow to waste to direct an unwanted section of the chromatographic injection away from the MS source.
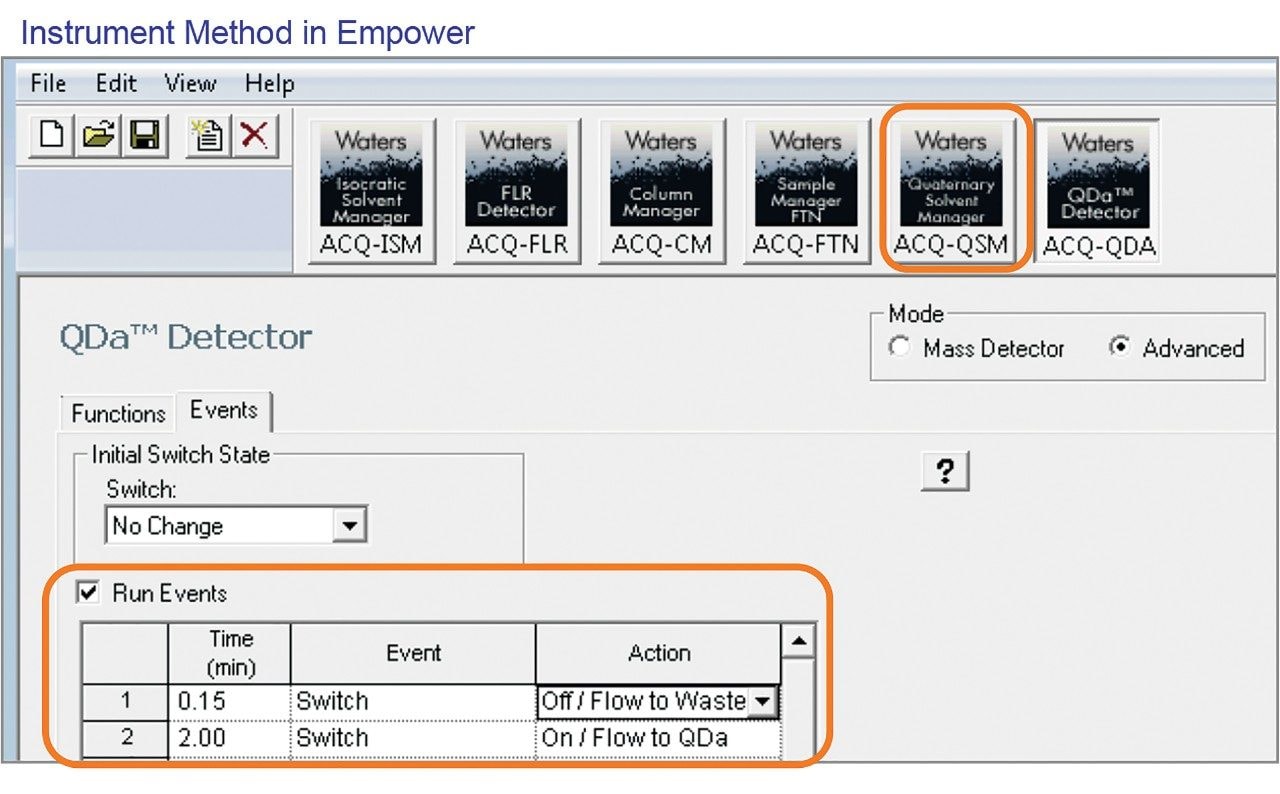
For the analysis of potentially genotoxic impurities, methyl and ethyl esters of p-toluenesulfonic acid, we used the diverter valve to direct the API portion of the injection to waste. A sample solution containing 1 mg/mL of bretylium tosylate API was spiked with the esters at 0.1% and analyzed using an ACQUITY QDa Mass Detector. The MS TIC data without a diverter valve (Figure 1a) shows that API elutes before the analytes. Using a valve, we switched the flow of the mobile phase to waste at 0.15 minute to divert the API portion of the chromatographic injection away from the MS source (Figure 1b). At 2.00 minutes, we switched the flow back to the mass detector to analyze the esters. We programmed the instrument method of the ACQUITY QDa Mass Detector to automatically switch the valve to “ON / Flow to QDa” and “OFF / Flow to Waste” positions (Figure 2). For our method, the diverter valve was switched to an “OFF / Flow to Waste” position between 0.15 and 2.0 minutes to divert the flow into waste.
System suitability
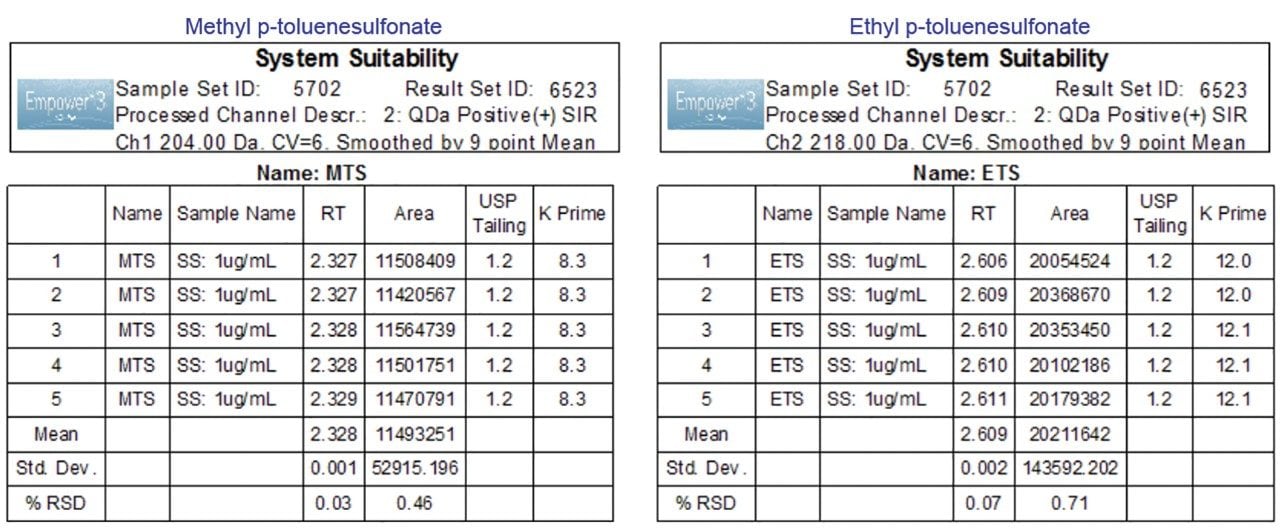
Performance of the MS method was verified by evaluating system suitability according to the specifications defined in the USP General Chapter, <621> Chromatography. System suitability results of five replicate injections of sample containing 1 μg/mL of methyl and ethyl esters are shown in Figure 3. The retention times and peak areas repeatability met the USP specifications of less than 2.0% RSD. The USP peak tailing is less than the USP recommendations of ≤1.5.
An ACQUITY Diverter Valve was used to divert an unwanted API portion of the chromatographic injection away from the source of the ACQUITY QDa Mass Detector to waste. The flow was redirected back to the mass detector for analysis of genotoxic impurities, methyl and ethyl esters of p-toluenesulfonic acid.
Diverting the sample matrix or a high concentration of API minimizes potential ionization enhancement/suppression and contamination of the mass detector, improving the accuracy and consistency of the results for the low-level detection of genotoxic impurities.
720005879, January 2017