Glycosylation is a complex and critical aspect of most therapeutic proteins that must be well characterized and monitored throughout product development and commercialization. RapiFluor-MS can be used to dramatically reduce sample preparation times and complexity, to enhance FLR sensitivity, and to dramatically improve MS sensitivity.
In this application note, we present the combined use of RapiFluor-MS labeling reagent, ACQUITY UPLC H-Class Bio System, and serial fluorescence/ACQUITY QDa Mass Detector for the monitoring of released N-glycan profiles from IgGs. Overall, this new workflow allows scientists to rapidly prepare samples, from glycoprotein to analysis in 30 minutes.
During the development of biopharmaceuticals, it is important to characterize and monitor glycoprofiles as they are often implicated as a product critical quality attributes due to their impact on safety, efficacy, and potency among other factors. It is well accepted that structural characterization of the glycoforms present is necessary, and that mass spectrometry (MS) often plays a large role in the identification of glycans.
Often, once the profile has been established, methods are transferred downstream which incorporate fluorescence detection. In many cases, there is a desire to obtain mass information for each detected peak even after characterization. These data have been difficult to obtain for a number of reasons, including a scarcity of mass spectrometers due to their cost and the requirement that MS specialized analysts are needed to generate meaningful and useful data.
In this application note, we present the combined use of Rapi Fluor-MS labeling reagent, ACQUITY UPLC H-Class Bio System, and serial fluorescence/ACQUITY QDa Mass Detector for the monitoring of released N-glycan profiles from IgGs. Overall, this new workflow allows scientists to rapidly prepare samples, from glycoprotein to analysis in 30 minutes.
In addition, Rapi Fluor-MS labeling yields unprecendented MS response,1 which enables the use of the ACQUITY QDa for mass detection. We will discuss the improved sensitivity and charge state profile afforded by Rapi Fluor-MS, its general utility for fluorescence and mass detection, and the quality of ACQUITY QDa mass spectra obtained for a range of IgG glycan structures.
|
LC system: |
ACQUITY UPLC H-Class Bio |
|
Detectors: |
ACQUITY UPLC FLR and ACQUITY QDa Mass Detector |
|
Column: |
ACQUITY UPLC Glycan BEH Amide, 130Å, 1.7 μm, 2.1 x 150 mm (p/n 186004742) |
|
Column temp.: |
60 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
2 μL |
|
Data rate: |
5 points/sec |
|
Excitation wavelength: |
265 nm |
|
Emission wavelength: |
425 nm |
|
Sample rate: |
5 points/sec |
|
Mass range: |
500–1250 Da |
|
Cone voltage: |
15 V |
|
Capillary voltage: |
1.5 kV |
|
Probe temp.: |
500 °C |
|
Ionization mode: |
ESI+ |
|
Mobile phase A: |
Acetonitrile (Pierce, LC-MS Grade) |
|
Mobile phase B: |
50 mM ammonium formate, pH 4.4, (LC-MS grade, Waters Ammonium Formate Concentrate) |
|
Mobile phase C: |
Acetonitrile (LC-MS grade) |
|
Mobile phase D: |
Acetonitrile (LC-MS grade) |
|
Time |
Flow rate(mL/min) |
%A |
%B |
%C |
%D |
|---|---|---|---|---|---|
|
Initial |
0.400 |
75 |
25 |
0 |
0 |
|
35.0 |
0.400 |
54 |
46 |
0 |
0 |
|
36.5 |
0.200 |
0 |
100 |
0 |
0 |
|
39.5 |
0.200 |
0 |
100 |
0 |
0 |
|
42.5 |
0.200 |
75 |
25 |
0 |
0 |
|
47.4 |
0.400 |
75 |
25 |
0 |
0 |
|
55.0 |
0.400 |
75 |
25 |
0 |
0 |
SYNAPT G2-S was used for assessment of Rapi Fluor-MS versus 2-AB N-glycan charge states. See Reference 1 for experimental details.
The Rapi Fluor-MS Glycan Performance Test Standard (p/n 186007983) was reconstituted in 25 μL of a mixture of DMF/acetonitrile/water at a ratio of 22.5%:55.5%:22%, respectively and used directly. For each analysis the injection volume was 2 μL, which corresponds to 32 pmol of released and labeled N-glycan on column. LC/MS-grade acetonitrile and water were purchased from Pierce. Ammonium formate was prepared using Waters Ammonium Formate Solution-Glycan Analysis (p/n 18600708) by pouring the entire contents of the solution into 1 L of water and mixed. The UPLC System used was dedicated for applications which do not require non-volatile salts to reduce the likelihood of adduct formation in the mass detector.
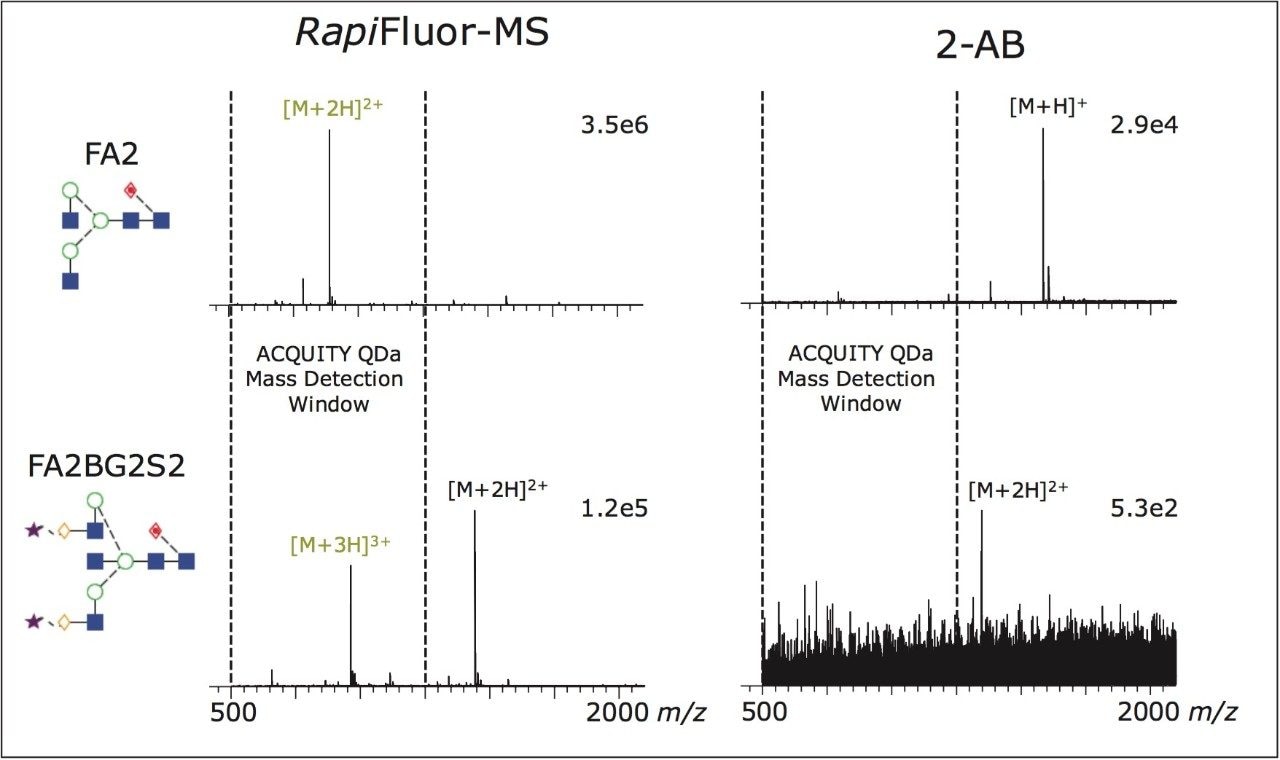
Addition of mass detection to an existing analytical workflow permits rapid and unambiguous identification of glycans. Historically, this has been a difficult task due to the need for high resolution instruments with appropriate sensitivity to obtain meaningful mass data. To overcome this issue, the novel labeling reagent, Rapi Fluor-MS, can been used. Rapi Fluor-MS dramatically increases both the MS sensitivity and charging of released N-glycans.
To demonstrate this, we compared the mass spectra of Rapi Fluor-MS labeled glycans to those of glycans labeled with a more traditional fluorescent label, 2-AB. This analysis was performed using timeof- flight mass spectrometry, which characteristically has a very wide mass range. The charge state characteristics of the different labeling technologies could thereby be objectively observed.
As shown in Figure 1, signal intensity improves dramatically when using Rapi Fluor-MS. Equally interesting is the shift in the charge states of the detected glycan ions that results from use of Rapi Fluor-MS labeling. As shown, Rapi Fluor-MS labeled FA2 near exclusively adopts an [M+2H]2+ charge state, while more complex structures begin to adopt even higher [M+3H]3+ charge states. In each case, at least one highly populated charge state falls well within the mass range of the ACQUITY QDa. Accordingly, Rapi Fluor-MS makes it feasible to use the cost effective, user-friendly ACQUITY QDa Mass Detector for N-glycan monitoring experiments.
As discussed above, routine detection of N-glycans with the ACQUITY QDa is made possible by Rapi Fluor-MS labeling. Importantly, ACQUITY QDa mass detection can be paired with fluorescence detection to facilitate obtaining optical-based quantification along with corroborating data on peak homogeneity and mass information. To enable this data to be collected routinely, the design characteristics of the ACQUITY QDa are such that users without extensive mass spectrometry training are able to generate meaningful mass data easily.
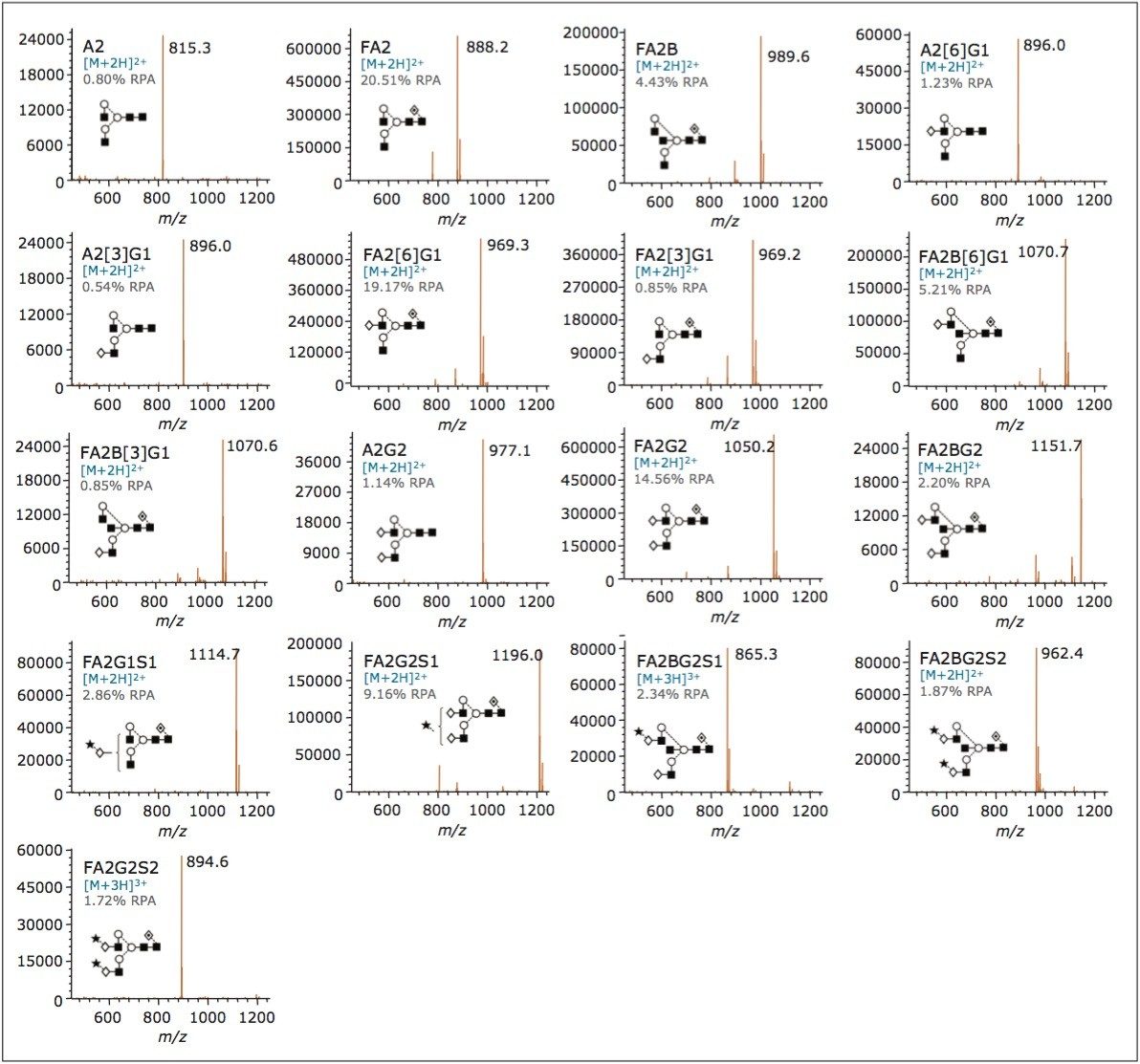
To demonstrate this ability, we separated a sample of IgG released N-glycans labeled with Rapi Fluor-MS and monitored the eluting glycans with both FLR and ACQUITY QDa detectors. As shown in Figure 2, high quality data were obtained for both detector channels, with each species identified in the FLR also represented with ACQUITY QDa MS data such that peak assignments can be readily confirmed. Within Empower Software, it is possible to annotate peaks with component and mass information, which makes reviewing data simple, as exemplified in Figure 2.

While the ability to detect N-glycan structures with the ACQUITY QDa is impressive, spectral quality is paramount for N-glycan monitoring, particularly when there is a need to interrogate the data in detail. We therefore reviewed the quality of MS data associated with peaks observed in the previously shown chromatograms. Figure 3 illustrates the spectra for each assigned peak in Figure 2. Notice that the ACQUITY QDa produced clean, easily interpretable mass spectra for the Rapi Fluor-MS labeled glycans, regardless of their relative abundance, molecular weight, or sialic acid content. Clearly, the ACQUITY QDa together with Rapi Fluor-MS can provide highly informative data that can be used to increase the confidence of assignments made during routine detection of N-glycans.
Glycosylation is a complex and critical aspect of most therapeutic proteins that must be well characterized and monitored throughout product development and commercialization. As discussed in this application note, Rapi Fluor-MS can be used to dramatically reduce sample preparation times and complexity, to enhance FLR sensitivity, and to dramatically improve MS sensitivity. By improving glycan MS sensitivity, Rapi Fluor-MS labeling permits the use of mass detection with the ACQUITY QDa and thereby affords greater confidence in peak monitoring across the range of structures encountered during biopharmaceutical development.
Taken together, Rapi Fluor-MS labeling and HILIC-FLR-MS with the ACQUITY UPLC H-Class Bio System and the ACQUITY QDa Mass Detector offer an unparalleled solution for monitoring the N-glycan profiles of biotherapeutics.
720005352, March 2015