This work demonstrates a rapid, simple and powerful approach to genotoxic impurity identification at the Threshold of Toxicological Concern (TTC) using the Xevo TQD when used in a qualitative manner with the ASAP Probe and product ion confirmation (PIC).
A rapid, simple and powerful approach to genotoxic impurity identification at the Threshold of Toxicological Concern (TTC) using the Xevo TQD when used in a qualitative manner with the ASAP Probe and product ion confirmation (PIC).
Alkyl sulfonic acids, particularly methanesulfonic acid, benzenesulfonic acid, and p-toluenesulfonic acid, are a common class of alkylating agents used in the pharmaceutical industry as alkylating reagents, catalysts, and in purification steps in the chemical synthesis of an API. In addition, these sulfonic acids are often used as the final salt form of the drug due to improved chemical properties or bioavailability.
The presence of any residual alcohols from synthetic reaction or re-crystalization steps may result in the formation of alkyl esters of the sulfonic acids. Many of these mesylate, besylate, or tosylate esters are known to be genotoxic while others are potentially genotoxic, requiring monitoring in the drug substance and drug product.
Typical methods utilized in the past for the analysis of these akyl sulfonate esters have been based on GC-MS or HPLC-UV/MS, with derivatization typically using run times in the order of 20 to 30 minutes. We have previously demonstrated how good results can also be achieved using UPLC-MS with run times of less than five minutes.
In this application note, we show how the presence of genotoxic impurities at Threshold of Toxicological Concern (TTC) can be quickly and easily detected using the Xevo TQD, a tandem quadrupole detector, with an Atmospheric Pressure Solids Analysis Probe (ASAP Probe).
The Xevo TQD was tuned to each of the three impurity standard solutions using the on-board fluidics and IntelliStart allowing the instrumental conditions for the tablet analysis to be chosen quickly and easily.
|
MS system: |
Xevo TQD |
|
Polarity: |
API+ |
|
Corona: |
0.50 μA |
|
Corona: |
1.5 kV |
|
Cone: |
30.00 V |
|
Extractor: |
3.00 V |
|
Source temp.: |
150 °C |
|
Probe temp.: |
450 °C |
|
Desolvation gas flow: |
400 L/Hr |
|
Polarity: |
API- |
|
Corona: |
0.80 μA |
|
Corona: |
1.5 kV |
|
Cone: |
30.00 V |
|
Extractor: |
3.00 V |
|
Source temp.: |
150 °C |
|
Probe temp.: |
450 °C |
|
Desolvation gas flow: |
400 L/Hr |
A single 10-mg amlodipine besylate tablet was crushed and solubilized in 5 ml of acetonitrile. The supernatant was removed and diluted 1:1 with water to give a 1 mg/mL solution of amlodipine besylate. Standard solutions of methyl- and ethyl- benzene sulfonates and ethyl-toluene sulfonate were prepared at 1 mg/mL in acetonitrile, and diluted to 15 µg/mL with water. These were spiked into the 1 mg/mL amlodipine solution using a 1:100 dilution, which equates to final impurities concentration of 1.5 µg (0.015%) per 10 mg tablet or the TTC when based on a single 10 mg per day dose of amlodipine besylate.
The ASAP probe was dipped into the spiked tablet solution, placed into the source and analyzed directly in multiple reaction monitoring (MRM) mode with product ion confirmation (PIC)1 enabled. The Xevo TQD can be used to perform quantification of a sample with simultaneous characterization of the MRM peak as it elutes from the chromatographic system – or as shown in this case the ASAP MRM peak.
This eliminates the need for separate injections when qualitative confirmation of MRM peaks is required and reduces the total analysis time in these situations. When used routinely, PIC increases user confidence in qualitative results from complex matrixes, and thus reduces the need for re-analysis.
The presence of each of the impurities was confirmed in the spiked tablet solution, and the identities of the impurities confirmed using PIC.
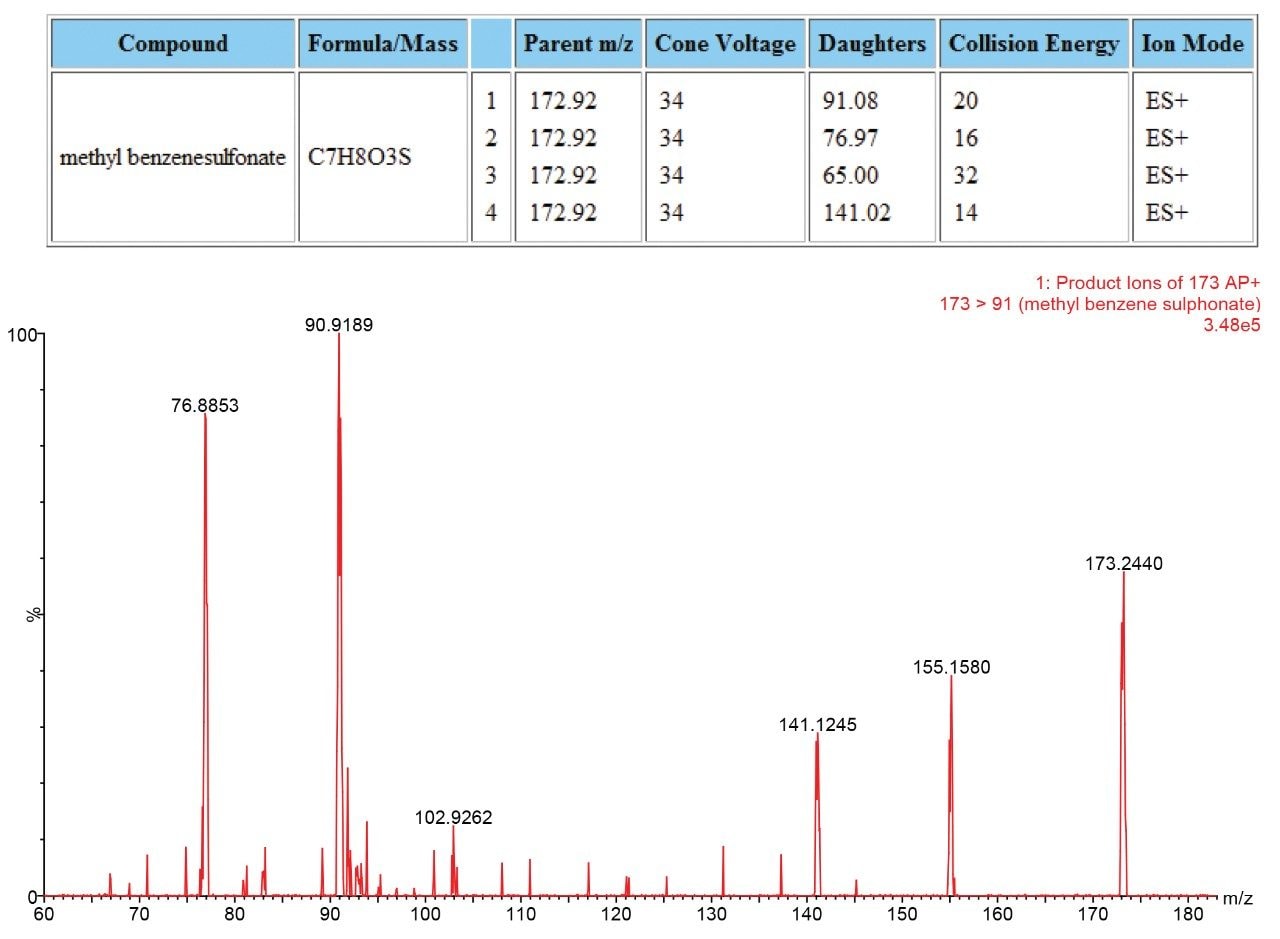
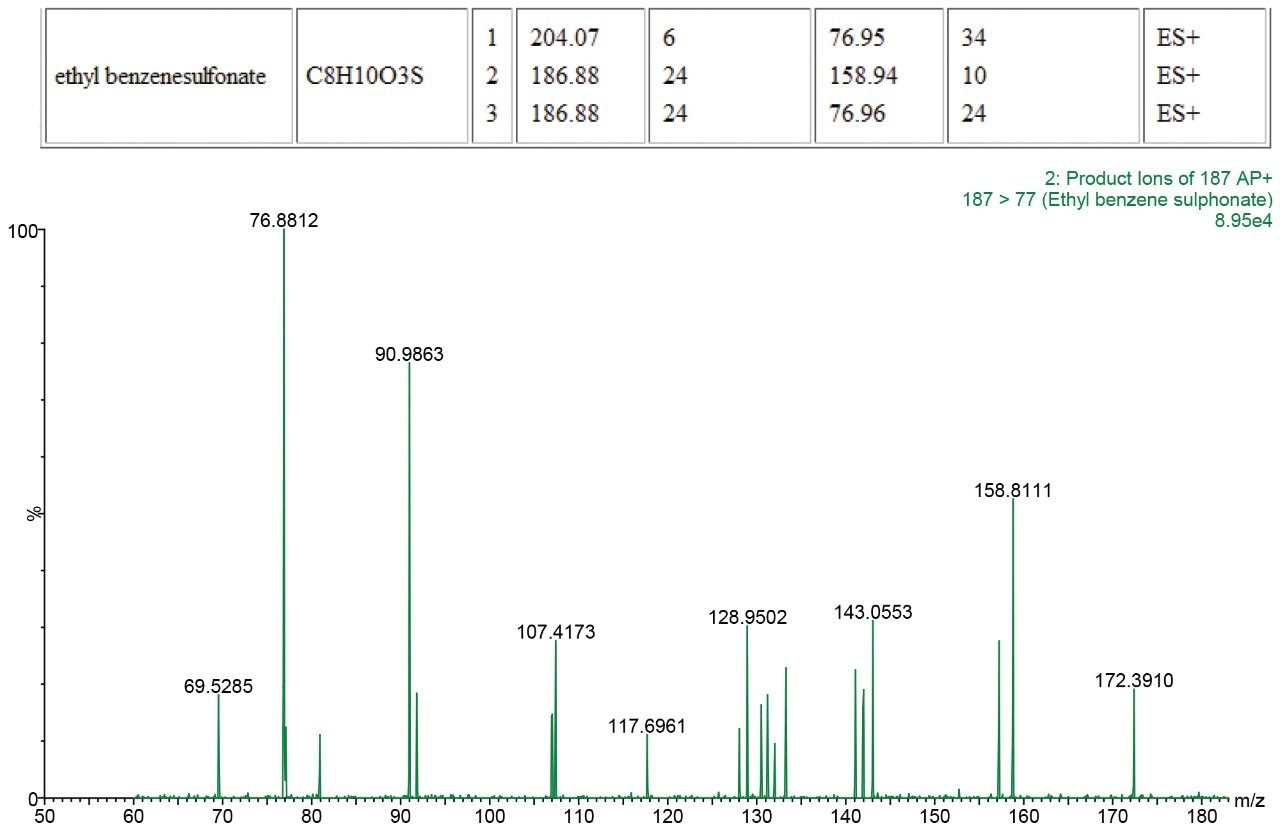
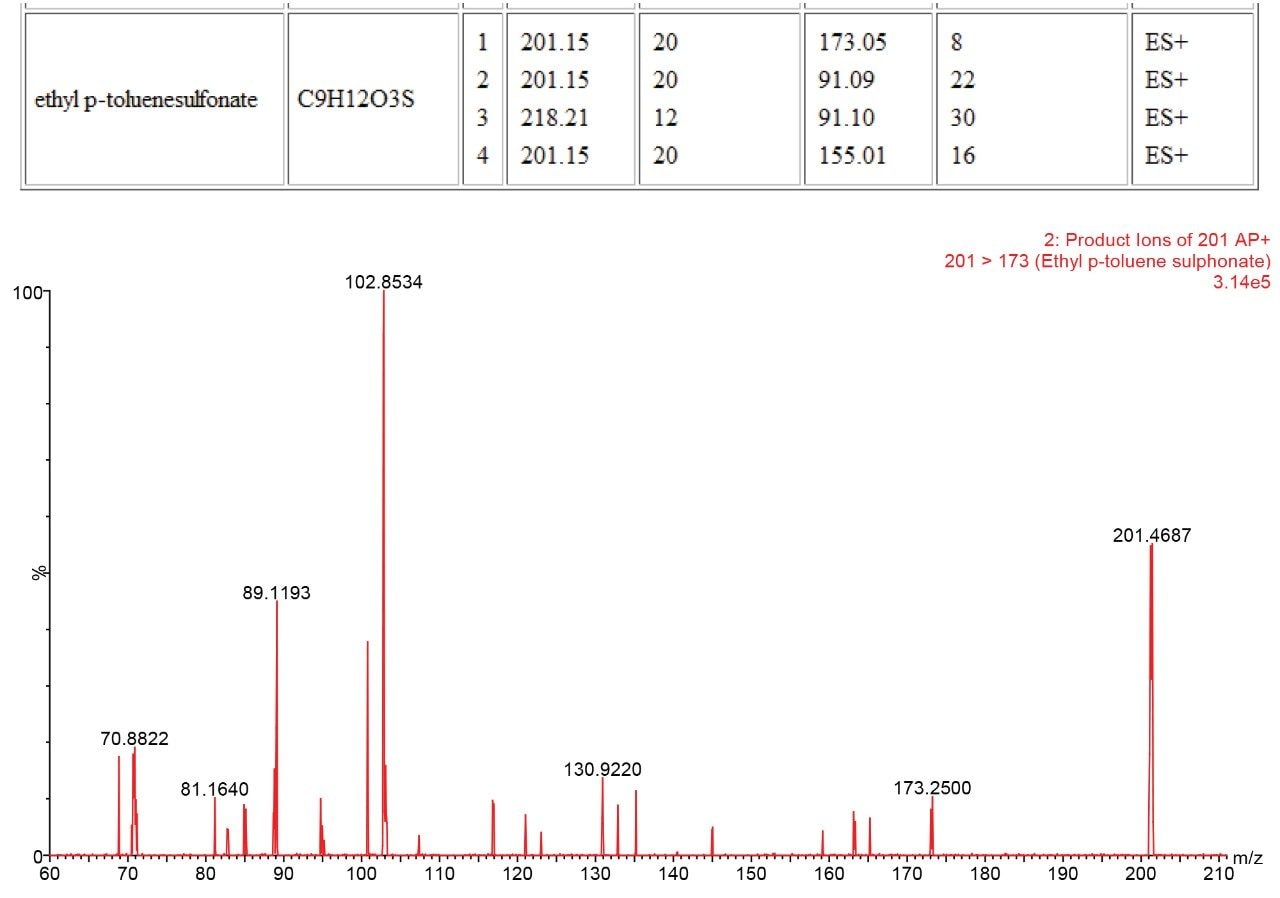
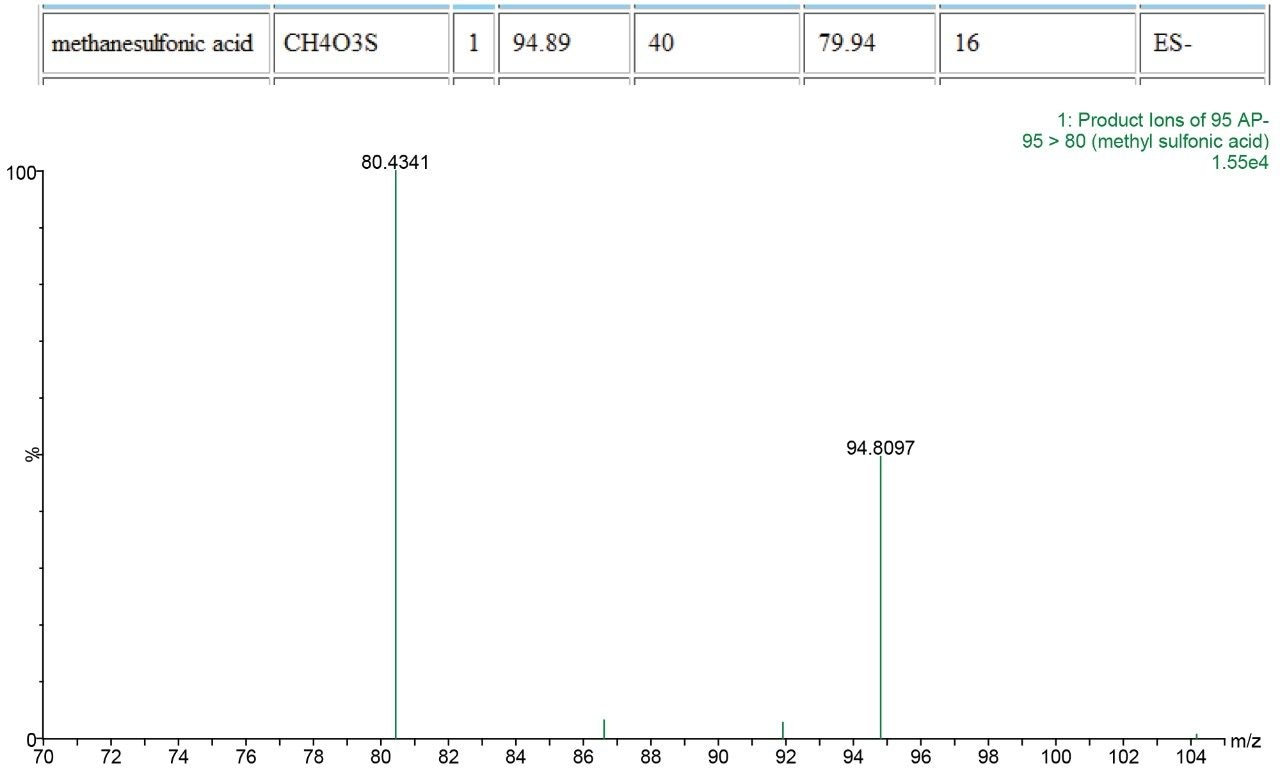
Genotoxic impurities at the Threshold of Toxicological Concern (TTC) can easily be identified using the Xevo TQD when used in a qualitative manner with the ASAP probe and PIC. This allows for rapid check for the presence of genotoxic and other identified impurities and also allows confirmation of identity through the use of PIC. This rapid, simple and powerful approach allows productivity gains into a routine laboratory setting that has not been possible before.
720004011, June 2011