
This is an Application Brief and does not contain a detailed Experimental section.
This Application brief demonstrates improved resolution and rapid analysis times achieved for the UPLC separation of aflatoxins M1, G2, G1, B2, and B1, without derivatization, using the ternary mixing capabilities of the ACQUITY UPLC H-Class System with the Waters Aflatoxin Analysis Kit.
Decrease analysis times for aflatoxins from 12 to 4.5 minutes with the ACQUITY UPLC H-Class System and automated ternary solvent blending – using an instrument that provides the operational familiarity of HPLC and the chromatographic performance of UPLC.
Aflatoxins are a group of mycotoxins produced as metabolites by the fungi Aspergillus Flavus and Aspergillus Parasiticus. They can be found in various foodstuffs such as grains, nuts, spices, and dairy products. There are four naturally occurring aflatoxins: B1, B2, G1, and G2. The third subset, M1, arise as a metabolic by-product when dairy cattle eat B1-contaminated grains. This can result in contaminated dairy products such as milk.
These compounds are toxic and can be carcinogenic to humans and animals. B1 and G1 are the more potent of the four naturally-occurring aflatoxins. Due to their toxicity, government regulatory agencies impose strict limits on aflatoxins in foodstuffs. For this reason, the food industry needs sensitive, accurate, and reproducible analytical methods to measure these compounds. The methods are usually based on reversed-phase HPLC with fluorescence detection. However, since reversed-phase eluents quench the fluorescence of aflatoxins B1 and G1, derivatization is commonly used to enhance the response of these analytes. Typical choices are pre-column derivatization with trifluoroacetic acid (TFA) or post-column derivatization with iodine, electrochemically-generated bromine, or photochemical UV.
These approaches are time consuming and require the purchase of costly postcolumn reactors, or, in the case of electrochemically-generated bromine, an electrochemical cell. In particular, the use of TFA raises concerns of handling safety by technicians.
Samples were prepared using the Waters Aflatoxin Analysis Kit featuring the VICAM AflaTest P methodology prior to analysis. Using the ACQUITY UPLC H-Class System with its UPLC-optimized fluorescence (FLR) Detector, a baseline separation of aflatoxins M1, G2, G1, B2, and B1 was achieved with a run time of 4.5 minutes. No derivatization was necessary. The ACQUITY UPLC H-Class System features a Quaternary Solvent Manager (QSM) and Auto•Blend Technology enabling dynamic, programmable blending of solvents. Here, a simple ternary mixture of water, methanol, and acetonitrile was applied in conjunction with an ACQUITY UPLC BEH C18 Column to perform the separation.
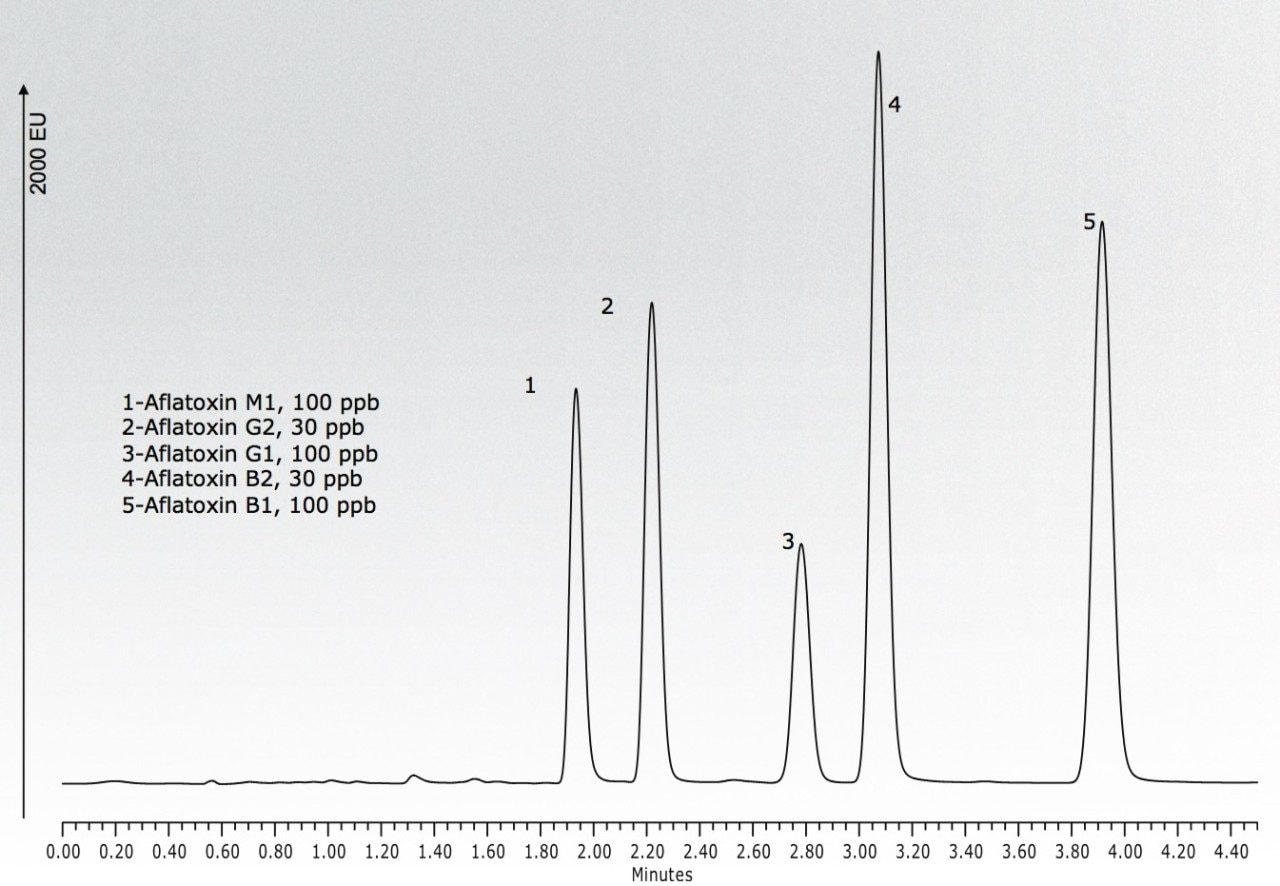
Two immediate benefits of using the ACQUITY UPLC H-Class System are its familiar operation compared to a traditional HPLC instrument, and, with UPLC capabilities, the system’s ability to decrease analysis time from 12 minutes to 4.5 minutes.
Eliminating a post-column derivatization system and post-column flow reduces band broadening, providing sharper peaks with higher signal-to-noise ratios. In turn, this enables more accurate integration and quantitation. Removing the need for derivatization also simplifies the system and reduces training, troubleshooting, and upkeep requirements. Additionally, using UPLC instead of HPLC reduced solvent consumption by 85 percent.
The ACQUITY UPLC H-Class System with the FLR Detector and the Aflatoxin Analysis Kit provides laboratories with the ability to reach the required level of sensitivity for quantifying aflatoxins without derivatization. The reduction in analytical run time offers faster sample turnaround times and improved efficiency.
720003286, January 2010