
A proprietary new procedure was developed for the manufacture of preparative columns with inner diameters of 19, 30, and 50 mm, and lengths from 30 to 250 mm. This new procedure results in columns with durable bed stability, better efficiency and higher loadability than other commercially available preparative columns.
In today’s drug purification environment, the demand for timely high purity results places huge emphasis on the integrity and stability of the preparative column. Complex sample starting materials demand high efficiency columns containing smaller particles (<10 μm) that was conventionally used for purification The challenge for the column manufacturer is to reproducibly produce analytical columns in preparative dimensions. We have developed an innovative procedure for the manufacture of preparative columns. To demonstrate the effectiveness of this new procedure, column resolution and bed capacities of an XTerra MS C18 Preparative Column was compared with two commercially available preparative columns.
Using this new, innovative process, a 19 x 100 mm column was packed with 5 μm XTerra MS C18 packing material. The two commercially available columns are also C18, 5 μm and are 21.2 x 100 mm in dimension. Econazole (3.2 mg/mL) and miconazole (3.2 mg/mL) were dissolved together in DMSO. This mixture of two basic compounds was used to examine the column separation efficiency. Imipramine (50 mg/mL) was dissolved in DMSO for bed capacity studies. The mobile phase consisted of A: water with 0.1% TFA, and B: acetonitrile with 0.1% TFA. A 10-min gradient from 95% A to 10% A was used. The flow rate was 18 mL/min for the 19 mm I.D. column, and 22.4 mL/min for the 21.2 mm I.D. columns. All experiments were run on the Waters AutoPurification System, which consists of a Waters 2525 Binary Gradient Module, a Waters 2767 Sample Manager, a Waters 2996 Photodiode Array Detector, and a Waters ZQ Mass Spectrometer. All experiments were conducted at ambient temperature.
Figure 1 is the comparison of the column resolution and stability between the XTerra MS C18 packed with the new, innovative process and two commercial C18 columns (Column A and Column B). Econazole and miconazole were selected based on their close retention times in the 10-min gradient. The total load of 6.4 mg is the same for all three columns. The resolutions for XTerra MS C18, Column A and Column B are 1.98, 1.43 and 1.24, respectively. The bed stability variances of 50 injections under the same loading show that the XTerra Column has a superior stability over the two commercial columns, which are either fluctuating or descending.
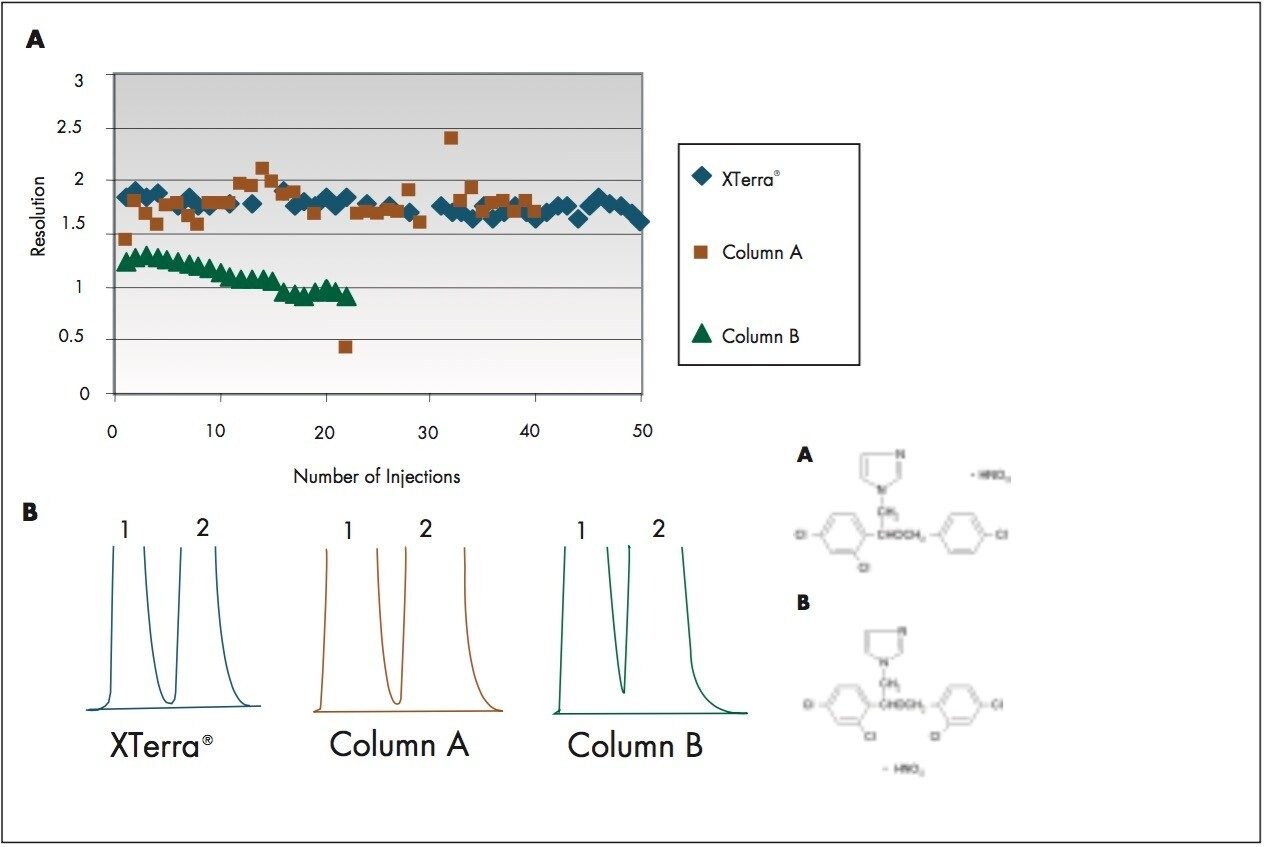
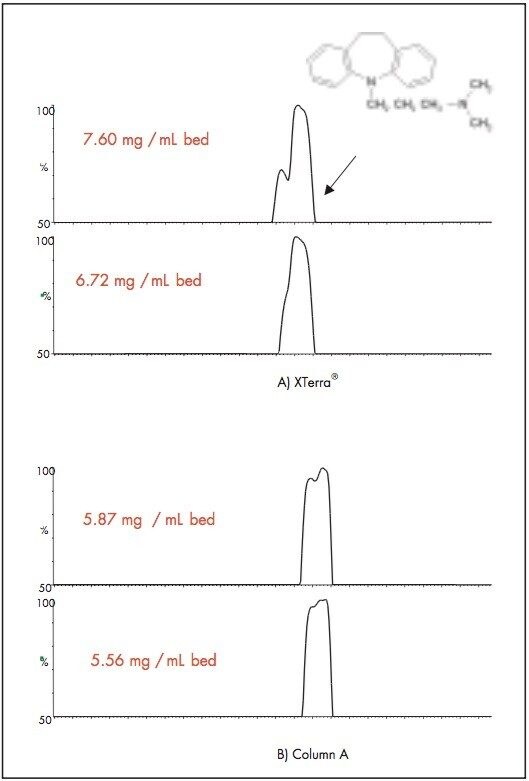
Figure 2 is the comparison of the bed capacity between the XTerra MS C18 with Column A. The same gradient was applied on both columns. An increasing volume of imipramine was injected onto the columns. The saturation bed capacity was reached when a shoulder or splitting peak was observed. As shown in Figure 2, XTerra MS C18 packed according to the new procedure has a higher loading per bed volume than Column A.
The new innovative procedure results in preparative columns with durable bed stability, better efficiency and higher loadability over the commercially available columns with similar stationary phases and the same particle sizes.
WA31815, June 2003