
For research use only. Not for use in diagnostic procedures.
Exact mass measured data, in both MS and MS/MS modes, have been used to characterize the metabolites of Ibuprofen found in human urine.
Using a hybrid quadrupole-time of flight mass spectrometer (Waters Micromass Q-Tof 2) it is possible to accurately measure the masses of fragment ions obtained during tandem mass spectrometry experiments and thereby predict their elemental compositions. Such information aids the elucidation of the structure of a precursor ion.
Previously, the difficulty in obtaining exact mass measurement of fragment ions has been the requirement for a reference mass in the observed spectrum. The mass of the precursor ion or of a known fragment is usually used but this requires prior information about the compound under investigation. To the best of the author's knowledge there is no method routinely in use that allows accuracy, to within 5 ppm, to be obtained on the fragment ions of a liquid chromatography/tandem mass spectrometry experiment, carried out on an uncharacterized precursor ion.
It has been recently demonstrated that the dualprobe LockSpray source (Figure 1 below) allows such an experiment to be performed.1

High spectral resolution is crucial to the ability of a mass spectrometer to accurately measure the mass to charge ratio (m/z) of an ion, enabling the separation of nominally isobaric species and allowing a more precise definition of peak centroid. In order to improve resolution the ToF analyzer of the Q-Tof 2 was operated in W-Optics mode,2 with the resultant increase in flight path providing resolution up to 17,500 FWHM.
Exact mass measured data, in both MS and MS/MS modes, have been used to characterize the metabolites of Ibuprofen found in human urine. Ibuprofen is a nonsteroidal anti-inflammatory drug that contains a chiral carbon in the propionic acid moiety of the molecule. The known biotransformations of Ibuprofen include hydroxylation, carboxylation, and glucuronidation.3
A control sample of human urine was obtained prior to administration of a commercially available ibuprofen capsule (200 mg). A second urine sample was taken after 1 hour. No sample extraction was performed and 2 μL of urine were directly loaded onto the HPLC column. A Waters Alliance 2695 Separations Module was used under the following conditions:
|
Flow rate: |
0.3 mL/min |
|
Column: |
Waters Symmetry C18 3.5 μm 2.1 x 100 mm |
|
Mobile phase A: |
10 mM aq. Ammonium formate |
|
Mobile phase B: |
Methanol |
|
Gradient: |
0% B at 0 mins = 25% B at 2 mins = 65% B at 22 mins |
TOF MS and MS/MS spectra, in negative electrospray, W-Optics mode, were acquired over the range 50 to 700 m/z. Argon was used as collision gas. Sulfadimethoxine (m/z 309.0658), at a concentration of 0.1 ng/μL, was introduced at a flow rate of 3 μL/min into the reference spray of the LockSpray interface. The reference spray was sampled once every 5 seconds.
Exact mass measured MS/MS spectra from the largest peaks at m/z 381 and m/z 397 (identified as potential metabolites by Metabolynx post-processing Software) are shown in Figures 2 and 3. Elemental composition assignments for the most intense fragment ions are shown inset.

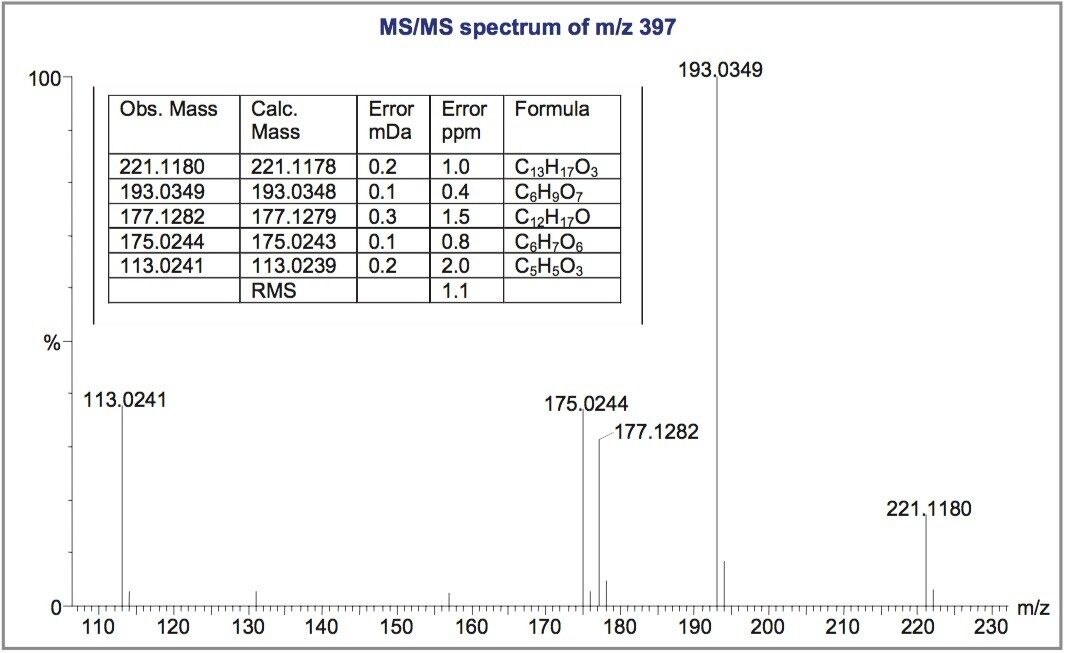
It can be seen that, even at the low values of m/z in question, the RMS error is less than 1.5 ppm in both cases. This excellent mass accuracy allows unambiguous assignment of the CID fragment peaks and a fragmentation scheme is postulated for the main metabolite at m/z 381 (Figure 4).
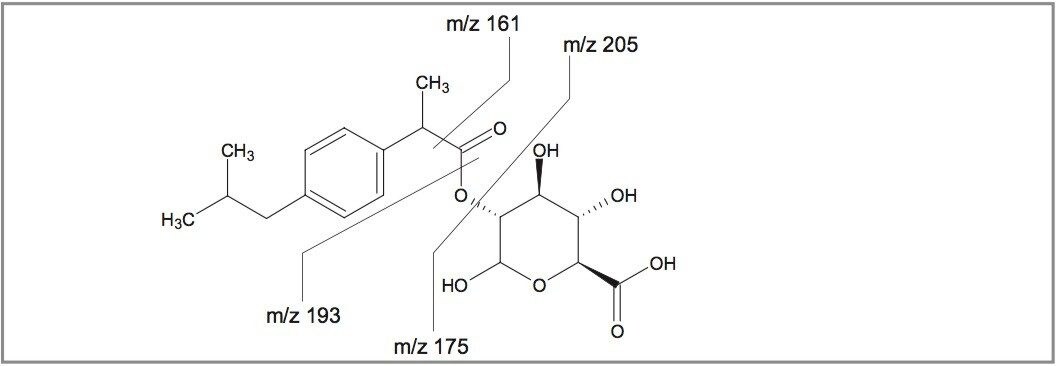
It may be seen that all of the peaks have been assigned. Some peaks have been assigned to more than one possible fragment structure (Figure 5). The ion at m/z 161 may either be part of the glucuronide moiety or it may be derived from the ibuprofen core. However, if the exact mass assignments shown are taken into account then the latter of these two options is the only possibility.
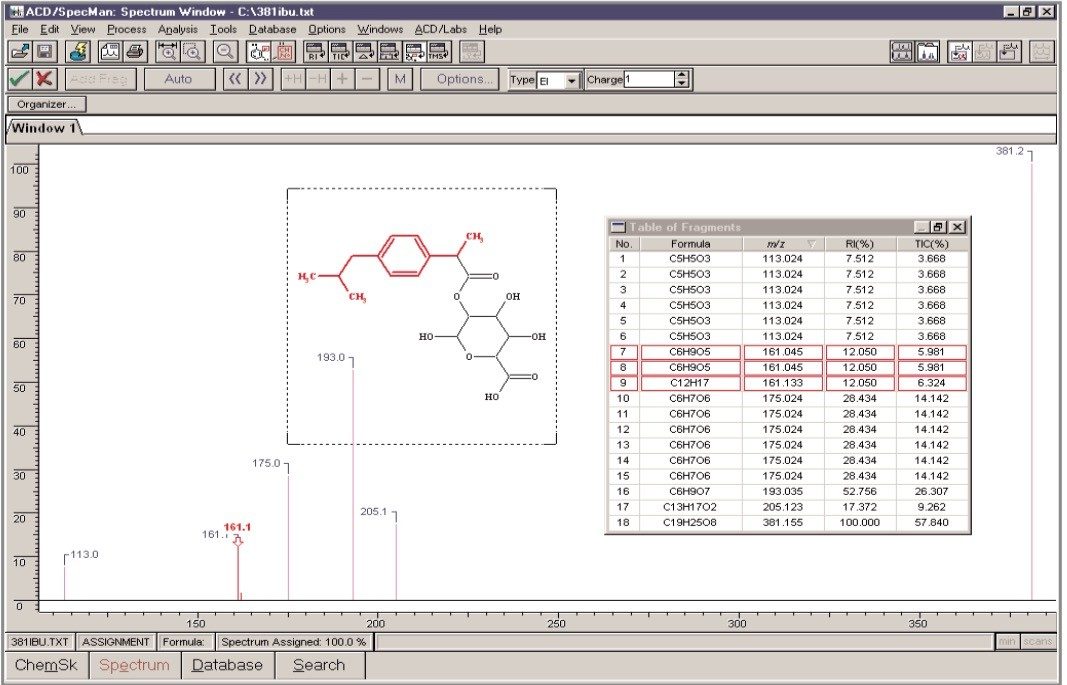
The ability to accurately measure the m/z of fragment ions is a significant aid to the structural elucidation process, particularly when dealing with complex biological matrices.
The Q-Tof 2 mass spectrometer, when operated with the LockSpray source and high resolution, is able to achieve <5 ppm RMS measurements, reducing the time and resources required for metabolite identification.
The ACD Labs SpecManager application, which may be accessed from within the MassLynx Software, is an extremely useful tool in the rapid interpretation of exact mass MS/MS spectra.
720000706, August 2003