Modernization of a Legacy Normal-Phase HPLC Method
Elom Pedanou, Lise Gauthier, Paula Hong
Waters Corporation, United States
Published on June 06, 2025
Abstract
Legacy methods developed during the early days of HPLC technology have not kept pace with recent advancements and modernizations in the field. Advancements in column chemistry and system robustness now allow for better separation and detection of analytes. In this study, the United States Pharmacopeia (USP) propofol assay normal-phase method was successfully modernized on the Alliance™ iS HPLC PDA System using a modern column. All systems met suitability requirements and demonstrated comparable results.
Benefits
- Seamless normal-phase method modernization on the Alliance iS HPLC PDA System
- XBridge™ Column technology for improved performance of USP propofol method
- Scaling column dimensions within USP guidelines for lower solvent consumption and reduced waste
Introduction
High performance liquid chromatography (HPLC) systems, particularly those within the pharmaceutical industry laboratories, play a crucial role in routine analysis. These systems, which are primarily used for reversed-phase chromatography, are also sometimes used with legacy separations such as normal-phase. Normal-phase chromatography is a challenging technique that is useful when separating polar and hydrophilic compounds. Many of the legacy normal-phase methods have not kept up with the pace of recent advancements and modernization especially with columns. Following the USP guidelines like General Chapter 621, a column used in a legacy method can be scaled and modernized.
In this study, the propofol assay from the USP is used to assess normal-phase modernization of a legacy column and migration of the method to the Alliance iS PDA System.1 The performance of the method will be evaluated by analysis of the system suitability requirements: Tailing factor, peak areas %RSD, and retention time %RSD.
Experimental
Sample Description
Reference standard was obtained from Sigma-Aldrich: Propofol (CAS No.: 2078-54-8). Standard solution consists of 2.4 mg/mL Propofol in hexane.
LC Conditions
|
|
|
|---|---|
|
LC system: |
LC system: |
|
LC system: |
Alliance iS HPLC PDA System |
|
Detection: |
Detection: |
|
Detection: |
Photodiode array detector, 10 mm Analytical flow cell, 275 nm@10 points/second |
|
Vials: |
Vials: |
|
Vials: |
TruView™ pH Control LCMS Certified Max Recovery Vials, p/n: 186005662CV |
|
Columns: |
Columns: |
|
Columns: |
1. Spherisorb™ 5 µm Silica Column, 80 Å, 4.6 × 200 mm (p/n: PSS830114) 2. XBridge BEH™ HILIC Column, 130 Å, 5-µm, 4.6 mm × 150 mm (p/n: 186004453) |
|
Column temperature: |
Column temperature: |
|
Column temperature: |
25 °C |
|
Sample temperature: |
Sample temperature: |
|
Sample temperature: |
10 °C |
|
Injection volume: |
Injection volume: |
|
Injection volume: |
10 µL |
|
Flow rate: |
Flow rate: |
|
Flow rate: |
2.0 mL/min |
|
Mobile phase: |
Mobile phase: |
|
Mobile phase: |
Hexane, acetonitrile, absolute alcohol (990:7.5:1) |
|
Method: |
Method: |
|
Method: |
Assay: Isocratic 10-min method |
Data Management
|
|
|
|---|---|
|
Chromatography software: |
Chromatography software: |
|
Chromatography software: |
Empower™ 3.8.1 |
Results and Discussion
Initial Column Issues
The propofol assay normal-phase USP method lists a column description of 4.6 × 200 mm L3 5-µm Column. An attempt to find a column that matched this description led to a Spherisorb 5 µm Silica 4.6 × 200 mm Column.
The Spherisorb column was shipped in a non-polar solvent and was thus ready for normal-phase use. The column was then equilibrated with the mobile phase for about 20 minutes before the analysis began. This included five replicate injections of the propofol standard solution to check system suitability. Once the chromatogram for the standard solution was analyzed, it was observed that there was some mass overloading of the column had occurred (Figure 2). This overloading may be observed as a large tailing factor as well as a high absorbance value.
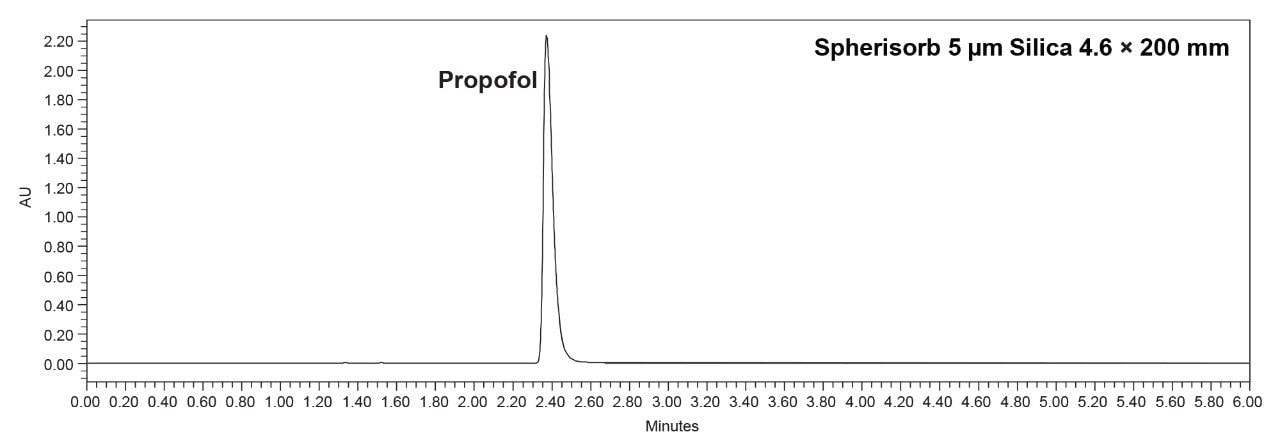
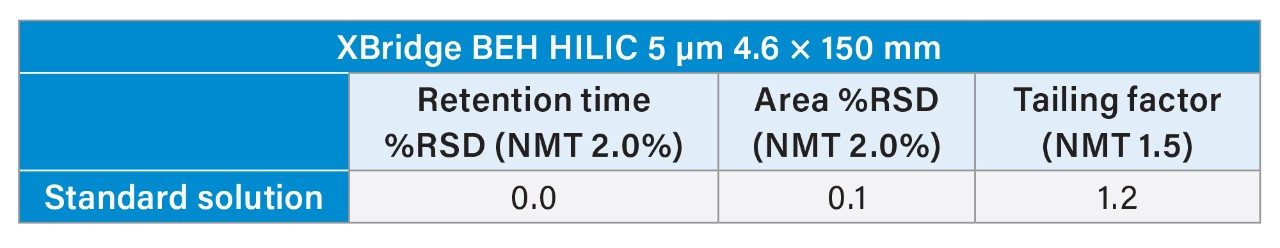
Further analysis of the standard solution showed that system suitability could not be fully met. As shown in Table 1, the tailing factor needs to be not more than (NMT) 1.5, but it was 1.8. However, the Spherisorb column was able to meet retention time and area %RSD of NMT 2.0% with values of 0.1% and 0.2%, respectively.
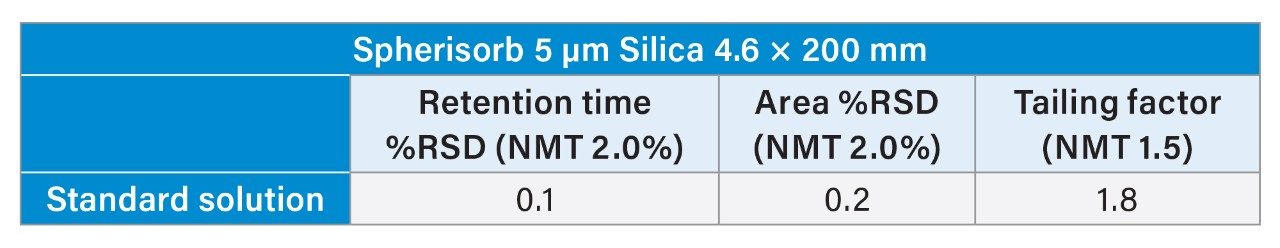
Due to the inability to meet all system suitability requirements, troubleshooting steps were taken to explore ways to help the analysis meet those requirements based on the assumption that some form of peak oversaturation may be the cause of the tailing issue.
Column Scaling and Modernization
Following the guidance from the USP General Chapter 621, column adjustments can be made if the ratio of the column length (L) to the particle size ratio (dp) remains within the specified limits of -25% to +50% of the prescribed L/dp ratio.2
The XBridge BEH HILIC column was selected as a feasible L3 column type for propofol analysis. This modern column chemistry is expected to minimize the oversaturation issues observed with the Spherisorb column. The BEH column has the same particle size of 5 µm and internal diameter of 4.6 mm, as specified in the propofol assay method. However, the available column length options for the BEH column do not include the 200 mm length specified by the method. Using the available information on column configurations for the BEH column and the specified column information in the method, the L/dp ratio was calculated to ensure the BEH column would meet the requirement. Using the Waters™ Columns Calculator (Figure 2), it was determined that the BEH column with a 150 mm length was suitable, as its L/dp ratio of 30,000 was within the acceptable range of the Spherisorb L/dp ratio of -25% to +50%.3
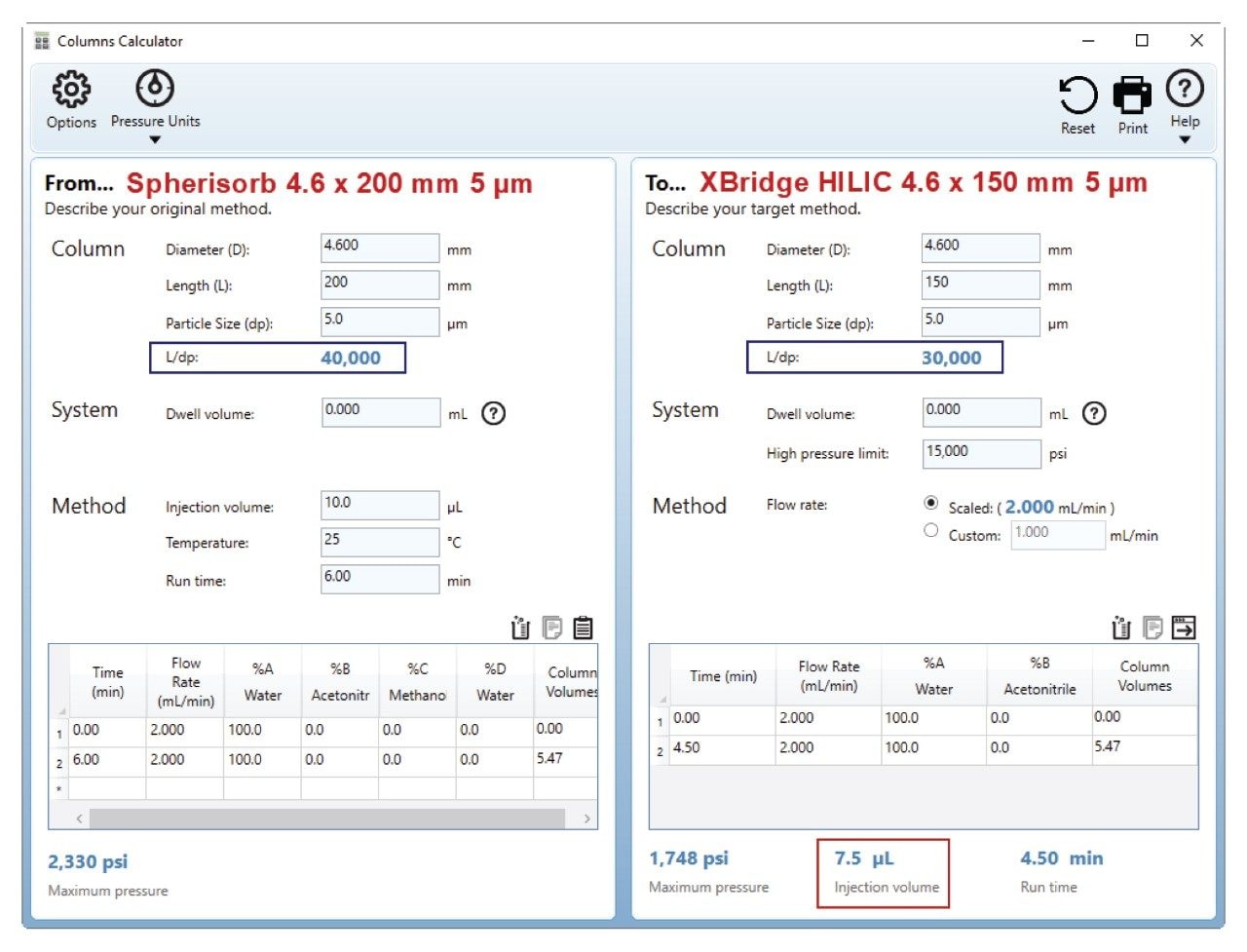
The USP General Chapter 621 also allows for the adjustment of the injection volume when the column dimensions are changed. Since the column calculator provided a new injection volume of 7.5 µL, this injection volume was selected for use as well.
Analysis with a Modern Column
The newly identified XBridge BEH HILIC column for the propofol assay analysis needed to be converted for use under normal-phase conditions, as it was shipped in polar solvents. Following the care and use guide for converting a column from polar to non-polar solvent usage, the column was equilibrated with the mobile phase before starting the assay analysis.
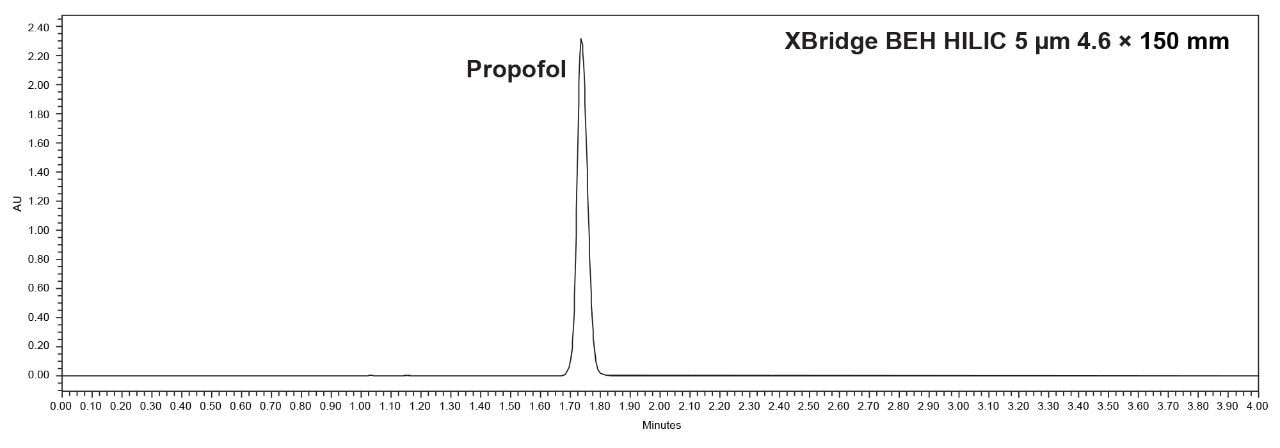
Upon analyzing the propofol peak after acquisition, no oversaturation issues that were present with the Spherisorb column were observed. The suitability requirements for retention time and peak area RSD% (NMT 2.0%) and the tailing factor (NMT 1.5) were met. As shown in Table 2, the tailing factor was 1.5, and the retention time and area %RSD were 0.0% and 0.1%, respectively.
Additional Benefit for Scaling
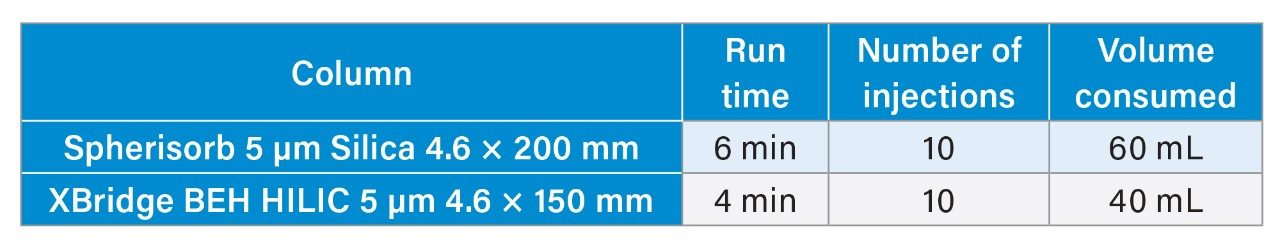
While the Spherisorb column offered a quick analysis time of around 6 minutes, switching to the XBridge column shortened the analysis time to about 4 minutes. This reduction not only cuts solvent usage by approximately 33.3% but also results in less waste production (table 3). These savings could potentially lower costs in running this analysis as well as disposing of the waste generated.
Conclusion
With advancements in liquid chromatography, modernizations in areas like column chemistry have become possible, guided by the USP. The propofol normal-phase assay method faced chromatographic issues and couldn't meet system suitability requirements with the Spherisorb column. However, replacing the Spherisorb column with a modern alternative like the XBridge BEH HILIC column on the Alliance iS HPLC PDA System resolved these issues, thus ensuring all system suitability criteria were met. This successful modernization, achieved by selecting a shorter and more suitable column, not only makes the analysis more environmentally friendly by reducing solvent consumption but also helps lower costs.
References
- Monograph: USP. Propofol. In: USP-NF. Rockville, MD: USP; 01 Mar 2024. DOI: https://doi.org/10.31003/USPNF_M70460_05_01.
- General Chapter: USP. 621 Chromatography, In: USP-NF. Rockville, MD: USP; 01 Dec 2024. DOI: https://doi.org/10.31003/USPNF_M99380_08_01.
- Waters Columns Calculator Version 2.0.
Featured Products
720008828, May 2025