Analysis of Free Inositol Stereoisomers in Dietary Supplements by Hydrophilic Liquid Chromatography using the Arc™ Premier System and ACQUITY™ QDa™ II Mass Detector
Jinchuan Yang, Stephanie Harden, Paul Rainville
Waters Corporation, United States
Published on December 24, 2025
Abstract
Inositol plays a key role in regulating numerous metabolic pathways and hormonal signaling processes. Inositol-based supplements are widely available and are commonly used to support hormone balance, alleviate symptoms of polycystic ovary syndrome (PCOS), and lower the incidence of gestational diabetes. Among the stereoisomeric forms of inositol, myo-inositol and D-chiro-inositol are the two predominant forms incorporated into nutraceutical formulations. Fast, reliable, and accurate analysis of these isomers is critical to ensure product quality, efficacy, and compliance.
The aim of this study was to develop an analytical procedure capable of simultaneously separating and quantifying inositol stereoisomers in dietary supplements. An existing Hydrophilic interaction liquid chromatography (HILIC) protocol employing a UPLC™ BEH™ Amide Column (1.7 µm, 2.1 mm x 150 mm) was systematically optimized on an Arc Premier System coupled with an ACQUITY QDa II Mass Detector. Chromatographic conditions, including mobile phase additives and column temperature, were optimized to achieve adequate resolution and reliable separation of seven inositol stereoisomers (allo-, muco-, epi-, D-chiro-, neo-, myo-, and scyllo-inositol).
Under the optimized conditions, all seven inositol stereoisomers were resolved in a 23-minute run using a binary gradient elution comprising ACN-water mixtures (A: 90:10 v/v, 0.01% NH4OH; B: 50:50 v/v, 0.01% NH4OH, 20 mM NH4HCO3). A minimum resolution of 1.6 was achieved for the critical pair (allo-/muco-inositol). Excellent analytical performance in linearity (R2 >0.998), sensitivity (ranged from 0.09 to 0.9 ppm), precision (relative standard deviation (RSD) less than 1.1%), and accuracy (recovery between 98% to 106%) have been demonstrated for myo-inositol and D-chiro-inositol in commercial supplements. This optimized HILIC-mass spectrometry (MS) method offers a fast, reliable, and accurate solution for the routine quantification of inositol stereoisomers in dietary supplements.
Benefits
- Outstanding precision and accuracy in quantifying myo-inositol and D-chiro-inositol in dietary supplements using the Empower™ Chromatography Data System (CDS)-controlled ACQUITY QDa II Mass Detector
- Excellent method selectivity, effectively minimizing interference from structurally related sugars such as glucose, galactose, fructose, and mannose
- Reliable separation of seven inositol stereoisomers with a minimum resolution of 1.6 on an ACQUITY UPLC BEH Amide Column
Introduction
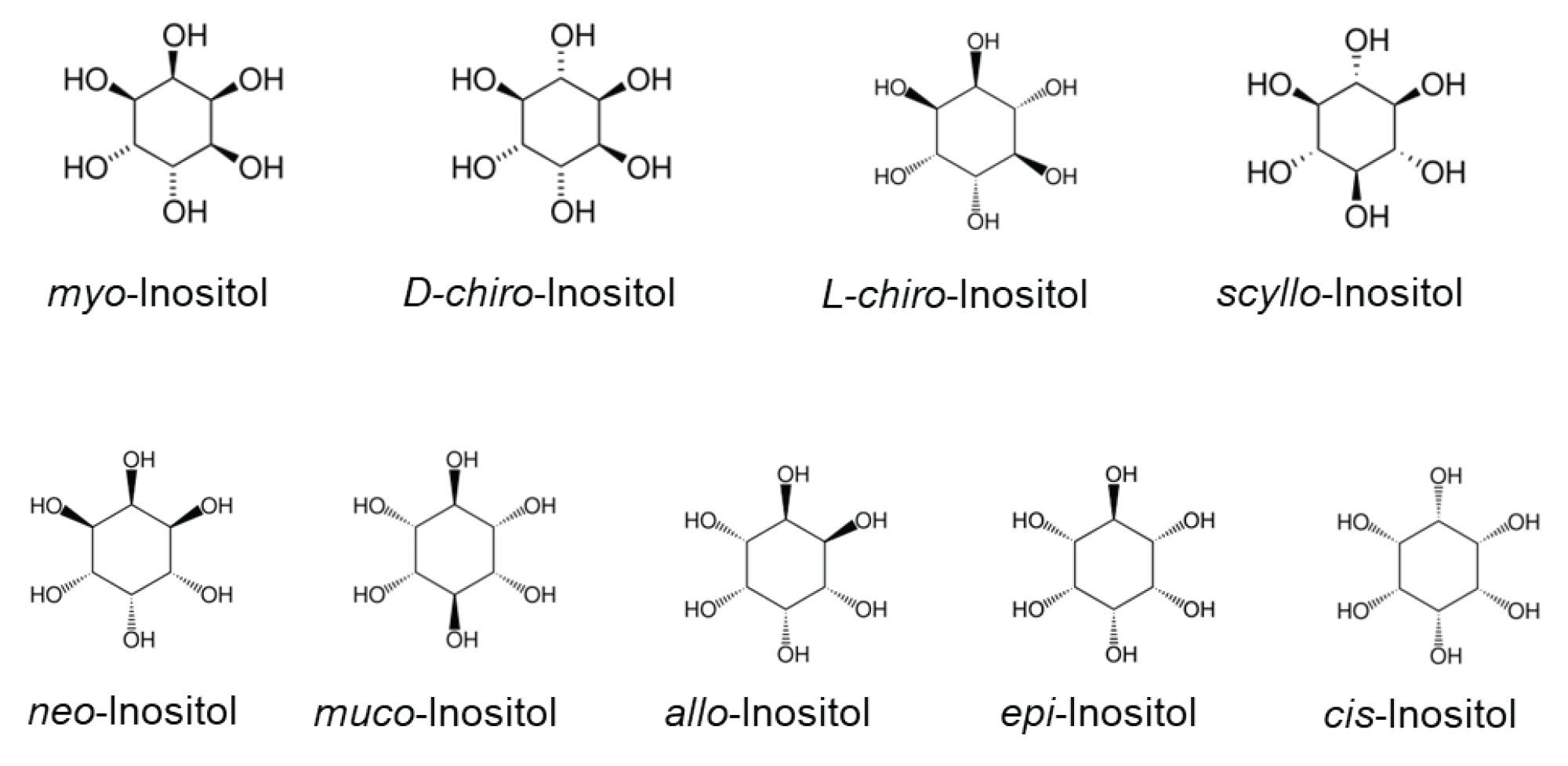
Inositols constitute a family of cyclic carbohydrates with a six-carbon ring structure and six hydroxyl groups (cyclohexane-1,2,3,4,5,6-hexol). There are nine stereoisomers: myo-, D-chiro-, L-chiro-, scyllo-, neo-, muco-, allo-, epi-, and cis-inositol. Figure 1 shows the structures of these stereoisomers. Myo-inositol and D-chiro-inositol are the two most abundant forms present in the human body, while other isomers, such as scyllo-, neo- and epi-inositol have also been detected in plasma and urine samples.1 The potential health benefits of inositol include managing insulin resistance, metabolic syndrome, PCOS, and gestational diabetes.2 Supplements that contain myo-inositol and D-chiro-inositol are widely available and commonly used to support hormone balance, alleviate symptoms of PCOS, and lower the incidence of gestational diabetes.3 Fast, reliable, and accurate analysis of these isomers in supplements is critical to maintain high product quality, ensure high efficacy, and meet compliance.
High-performance liquid chromatography (HPLC) and Gas chromatography (GC) are commonly employed for inositol analysis. GC provides high resolution for separating inositols; however, it requires derivatization and long run times. In contrast, HPLC offers rapid separation without the need for derivatization. HPLC-based standard methods are available for the determination of myo-inositol or D-chiro-inositol.4–6 However, no standard method is currently available for simultaneous determination of inositol stereoisomers. Fortunately, simultaneous analyses of stereoisomers of inositol by HPLC have been reported in literature,1,7,8 which provide a good starting point for developing a method for simultaneous analysis of inositol stereoisomers for routine testing applications.
The aim of this study was to develop an analytical procedure capable of simultaneously separating and quantifying free inositol stereoisomers in dietary supplements. An existing HILIC protocol employing a UPLC BEH Amide Column (1.7 µm, 2.1 mm x 150 mm) was optimized on an Arc Premier System coupled with an ACQUITY QDa II Mass Detector. Chromatographic conditions and mass detector parameters were systematically optimized. The method’s analytical performance in selectivity, linearity, sensitivity, precision, recovery, and robustness was assessed using standards and commercially available supplement samples. This study focused exclusively on free inositol isomers. Bound inositol was not within the scope of this study.
Experimental
Chemicals and Standards
Myo-Inositol (≥ 99%), D-chiro-inositol (≥98%), allo-inositol (≥97%), acetonitrile (ACN, LC-MS grade), ammonium hydroxide solution (NH₄OH, 28–30%), and ammonium bicarbonate (NH4HCO3, ≥99.5%) were purchased from Sigma-Aldrich (Milwaukee, WI). Epi-Inositol (≥98%), muco-inositol (≥95%), and neo-inositol (≥95%) were purchased from Biosynth International, Inc. (Gardner, MA). Scyllo-Inositol was purchased from Cayman Chemical Company Inc. (Ann Arbor, MI). Water (ultrapure, 18.2 MΩ•cm) was generated from a Milli-Q® System (IQ 7005) in the lab. Internal standard (ISTD) myo-inositol (1,2,3,4,5,6-D6, 98%) was purchased from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA).
Standard Preparation
Individual standard stock solutions of seven inositol stereoisomers (allo-, muco-, epi-, D-chiro-, neo-, myo- and scyllo-Inositol) were prepared in water at concentrations ranging from 1 to 3 mg/mL. The standard mix stock solution was prepared by mixing appropriate volumes of the seven individual standard stock solutions and diluting with water to obtain a final concentration of 50 - 150 µg/mL for each standard. The ISTD stock solution was prepared in water at a concentration of 1 mg/mL. All concentrations were recorded to three significant figures. Working standard solutions were prepared by mixing appropriate volumes of the standard mix stock solution, the ISTD stock solution, and water to achieve final concentrations of 0.05, 0.15, 0.5, 1.5, 5.0, and 15 µg/mL (or ppm). The ISTD concentration was kept at 10 ppm in these working standard solutions.
Sample Preparation
Dietary supplements from various brands were purchased via online stores. All supplements came in the form of capsules filled with powder contents. The powder contents were weighed and initially dissolved in water to prepare sample stock solutions at a concentration of 3 - 4 mg/mL. Heating at 50 °C for up to 30 minutes was applied when necessary. These sample stock solutions were then diluted tenfold with water to obtain sample intermediate I solutions, which were further diluted tenfold to produce sample intermediate II solutions. These sample intermediate II solutions were filtered through a 0.45 µm PVDF syringe membrane filter (p/n: WAT200827, Waters). Final sample solutions were prepared by mixing 50 µL of filtered sample intermediate II solution with 5 µL of ISTD stock solution and 445 µL of water in 1.5 mL Max Recovery Glass Vials (p/n: 186000327C, Waters). The ISTD concentration in these final sample solutions was kept at a concentration of 10 ppm.
Spiking Experiments
Myo-inositol and D-chiro-inositol were spiked into sample intermediate I solutions in various amounts and then analyzed the same way as samples. Spike recovery was assessed by subtracting the native inositol concentration from the total inositol measured and comparing that against the amount of inositol that was spiked and expressed in percentage.
LC Instrument and Conditions
|
UHPLC system: |
Arc Premier System equipped with a Quaternary Solvent Manager (QSM-R), a Sample Manager (FTN-R), a Column Manager, and coupled with an ACQUITY QDa II Mass Detector |
|
Software: |
Empower CDS |
|
Column: |
ACQUITY UPLC BEH Amide Column (1.7 μm, 2.1 mm x 150 mm, p/n: 186004802, Waters), or ACQUITY Premier BEH Amide VanGuard™ FIT Column (1.7 μm, 2.1 mm x 150 mm, p/n: 186009509, Waters) |
|
Column temprature: |
25 °C |
|
Run time: |
23 minutes |
|
Mobile phase A: |
ACN:water (90:10 v/v) with 0.01% NH4OH |
|
Mobile phase B: |
ACN:water (50:50 v/v) with 0.01% NH4OH and 20 mM NH4HCO3 |
|
Sample manager purge: |
ACN:water (50:50, v/v) |
|
Flow rate: |
0.35 mL/min |
|
Injuction volume: |
2 µL |
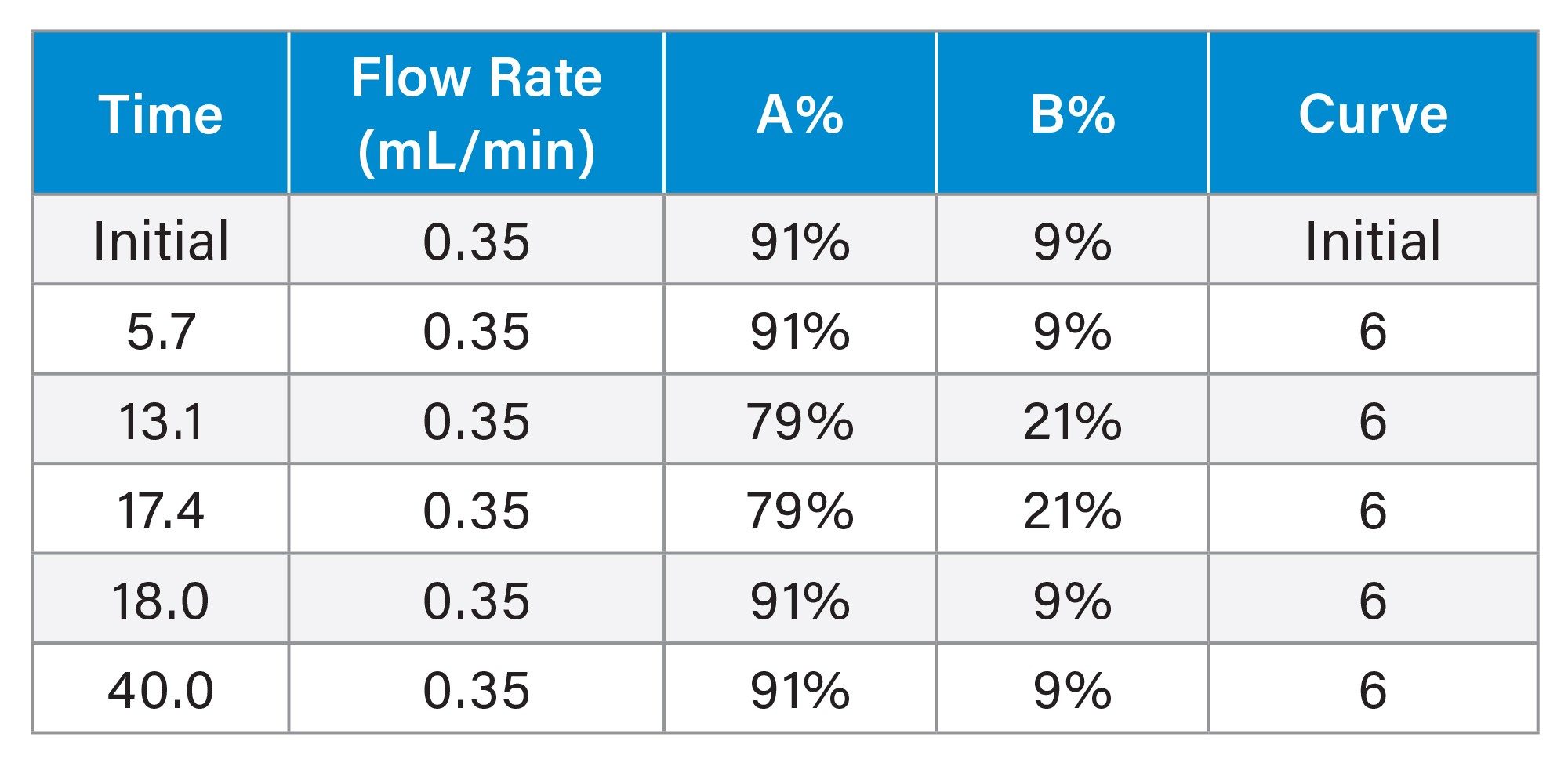
Gradient Elution Program
ACQUITY QDa II Mass Detector Parameters
|
Ionisation mode: |
ESI- |
|
Capillary voltage: |
0.5 kV |
|
Probe temperature: |
600 °C |
|
Cone voltage: |
7.0 V |
|
Sampling rate: |
5 Hz |
|
SIR [M]-: |
179 Da (for analytes) and 185 Da (for ISTD) |
Results and Discussion
Optimization of HILIC-MS Conditions
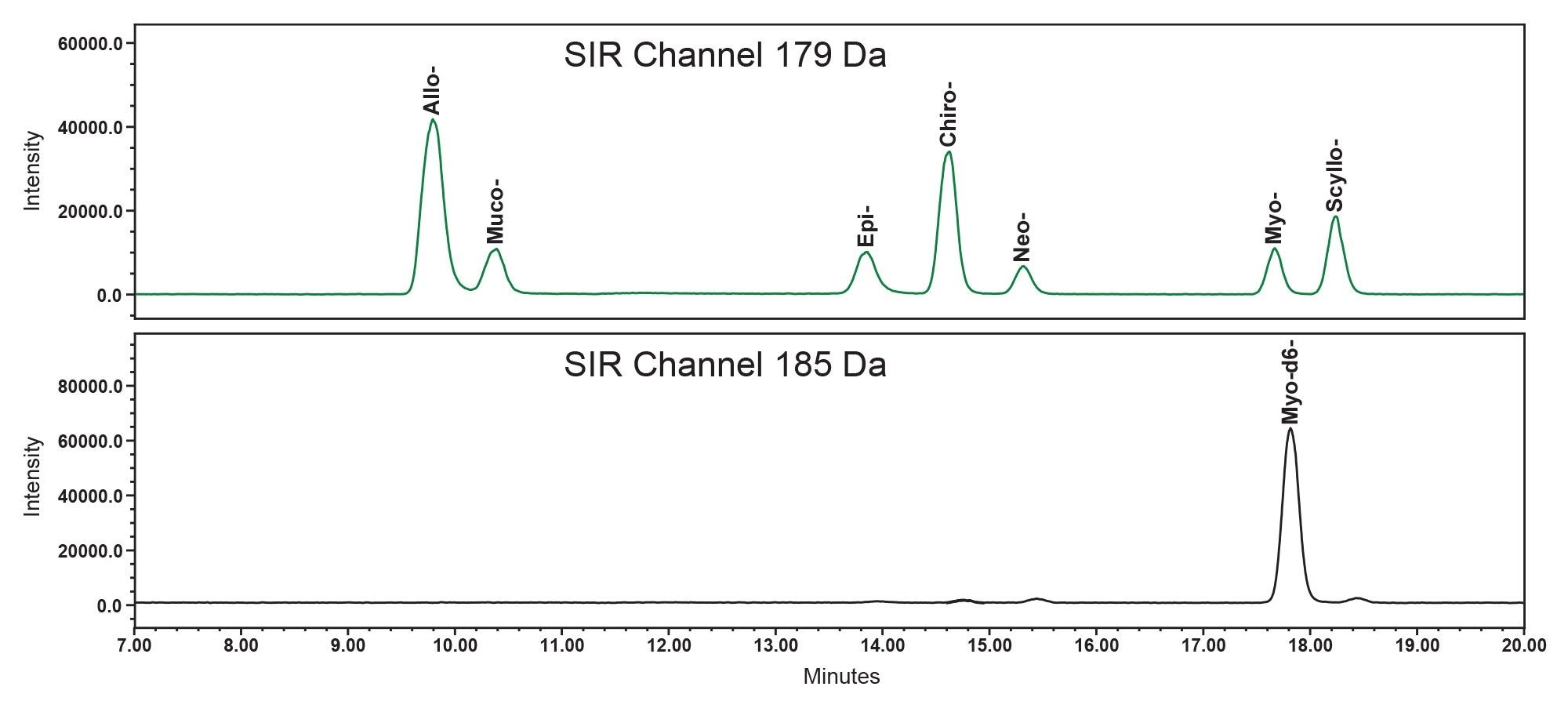
BEH Amide Columns have proven effective for the separation of inositol stereoisomers.1,7,8 In this study, the chromatographic conditions originally developed by Monnard et al1 were optimized to achieve improved resolution and more robust separation of seven inositol stereoisomers. Evaluation of column temperature effects in the range of 20 °C to 50 °C showed that optimal resolution was achieved at 25 °C. (Please note that the Column Manager can maintain column temperature up to 25 °C below ambient. For labs operating in hot climates, this Column Manager is highly recommended.) Increasing the column temperature negatively affected chromatographic resolution, and at 50 °C, a co-elution of D-chiro-inositol and neo-inositol was observed. The chromatographic resolutions between pairs of allo-/muco-, D-chiro-/Neo-, and myo-/scyllo-inositol were 1.6, 2.3, and 2.0, respectively (Figure 2). These results represented a substantial improvement over the original method, where the corresponding resolutions were 1.6, 1.3, and 1.3, respectively. Comparable or improved resolution was also achieved using another ACQUITY UPLC Amide Column from a different production lot and on an ACQUITY Premier BEH Amide VanGuard FIT Column (1.7 µm, 2.1 x 150 mm) (data not shown), confirming the robustness of the separation.
The capillary voltage and probe temperature of the ACQUITY QDa II Mass Detector were optimized. The optimal peak height of standards in selected ion recording (SIR) channel at 179 Da was achieved at a capillary voltage of 0.5 kV and a probe temperature of 600 °C, within the tested ranges of 0.3–0.8 kV and 300–600 °C, respectively.
Internal Standard
ISTDs are commonly employed in MS to compensate for variability during sample preparation, detection, and matrix effects for better accuracy and precision in quantification. In this study, the ISTD was primarily used to compensate for MS detection variation and matrix-related chromatographic interferences. Stable isotope-labeled standards were available only for myo-inositol; therefore, deuterated myo-inositol-d6 was employed as the ISTD for all inositol stereoisomers. Stable isotope labeled ISTD typically has the same retention time (RT) as the analyte. However, in this case, there was a notable RT shift between the myo-inositol-d6 and myo-inositol ( Figure 2). The RT shift could not be reduced across the temperature range (20 - 50 °C). This RT shift likely has to do with the slightly stronger hydrogen bonding of deuterium compared to hydrogen, which is one of the main interactions in HILIC. The RT shift did not impact quantification of myo-inositol and D-chiro-inositol in dietary supplements (see section Method Accuracy and Precision), indicating minimal matrix effects in these sample matrices.
Method Specificity
MS detection in the SIR mode (unit mass resolution) effectively eliminated potential interference from non-isobaric compounds. To assess interference from structurally related isobaric compounds such as glucose, galactose, fructose, and mannose, these compounds were analyzed under the optimized HILIC-MS conditions, and their RTs, peak shapes, and peak areas were evaluated. These compounds eluted as broad peaks at RTs prior to that of allo-inositol, which was the first peak (least retained) of inositol stereoisomers. Furthermore, their MS responses (peak area) were substantially lower than that of allo-inositol, less than 0.4% of the allo-inositol response at the same concentration level. These findings indicated that the analysis of inositol stereoisomers was unlikely to be affected by the presence of these monosaccharides in sample matrices.
Enhancing Separation Robustness
A gradual increase in column baseline backpressure was noted during the initial sample analysis using mobile phases containing 0.04% NH₄OH, the concentration used by Monnard et al.1 This problem was resolved by lowering the NH₄OH concentration to 0.01% and adding 20 mM NH₄HCO₃ to mobile phase B. Additional precautionary measures were implemented to maintain consistent and robust chromatographic performance. These measures included rinsing the column with 90% ACN (without additives) after completing sample analysis at the end of day and conditioning and equilibrating the column with five consecutive blank injections at the beginning of each set of sample analysis. Collectively, these optimization and preventive measures ensured stable column baseline backpressure and reproducible performance over an extended period.
Linearity and Limit of Quantification
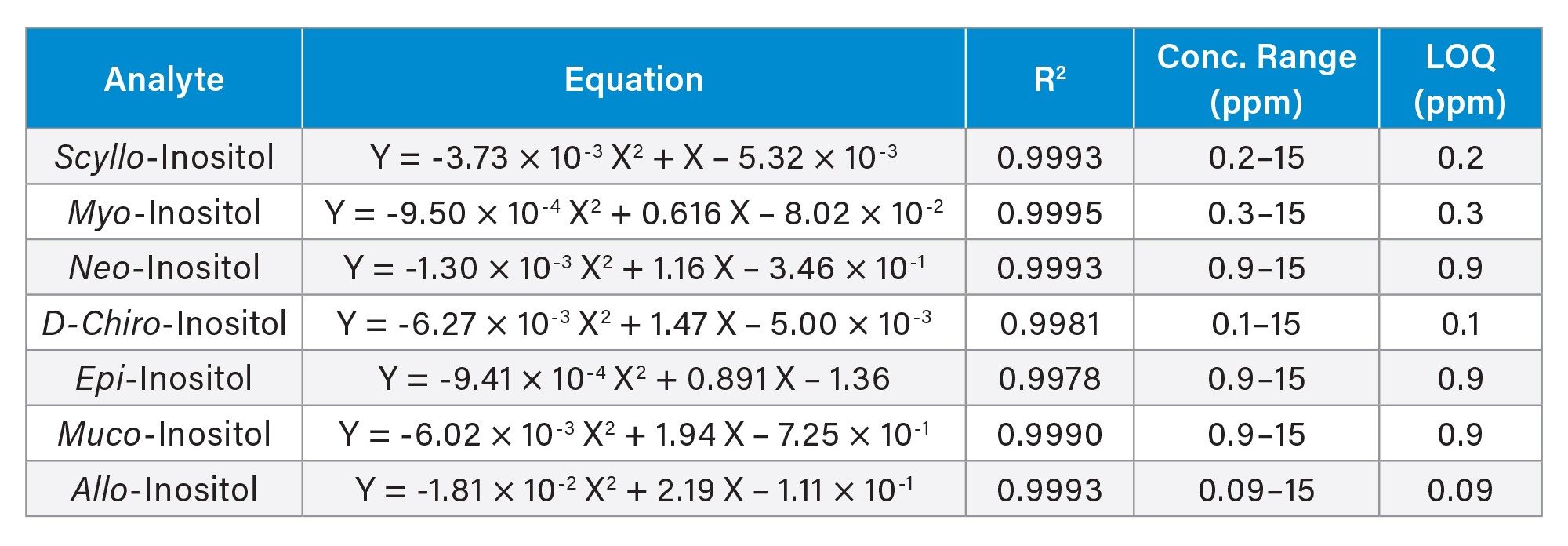
The relationship between the SIR responses (peak area ratio of standards to the ISTD) and standard concentrations was modeled using a second-order polynomial regression, fitted by the least-squares method with a 1/x weighting factor. The resulting calibration equations, coefficients of determination (R2), concentration ranges, and estimated limit of quantification (LOQ) for the seven inositol stereoisomers are presented in Table 1. LOQ were estimated based on a signal to noise at 10, using responses from low concentration standard solutions. All seven compounds exhibited excellent calibration linearity (R2 ≥ 0.998) and highly sensitive LOQs.
Method Accuracy and Precision
The accuracy and precision of the analytical method were assessed using spiking experiments. Myo-inositol and D-chiro-inositol were chosen for spiking because they are the most commonly used inositol forms in supplements. Table 2 summarizes the mean concentrations from triplicate measurements, RSDs, spiked concentrations, spiking levels (relative to native concentrations), RSDs for spiked samples, and recovery. The spiked myo-inositol levels ranged from 60% to 300% of the native concentration. The recovery for myo-inositol and D-chiro-inositol were between 101.1% to 106.0%, and 97.9% to 99.9%, respectively. These results fall well within the common ±10% limit. The RSDs for the determination of these two inositol isomers were below 1.1% for most of the samples, except for DS-3 and DS-4, where native D-chiro-inositol concentrations were below the LOQ. These results demonstrated outstanding accuracy and precision of this analytical method in quantifying inositol stereoisomers in dietary supplements.
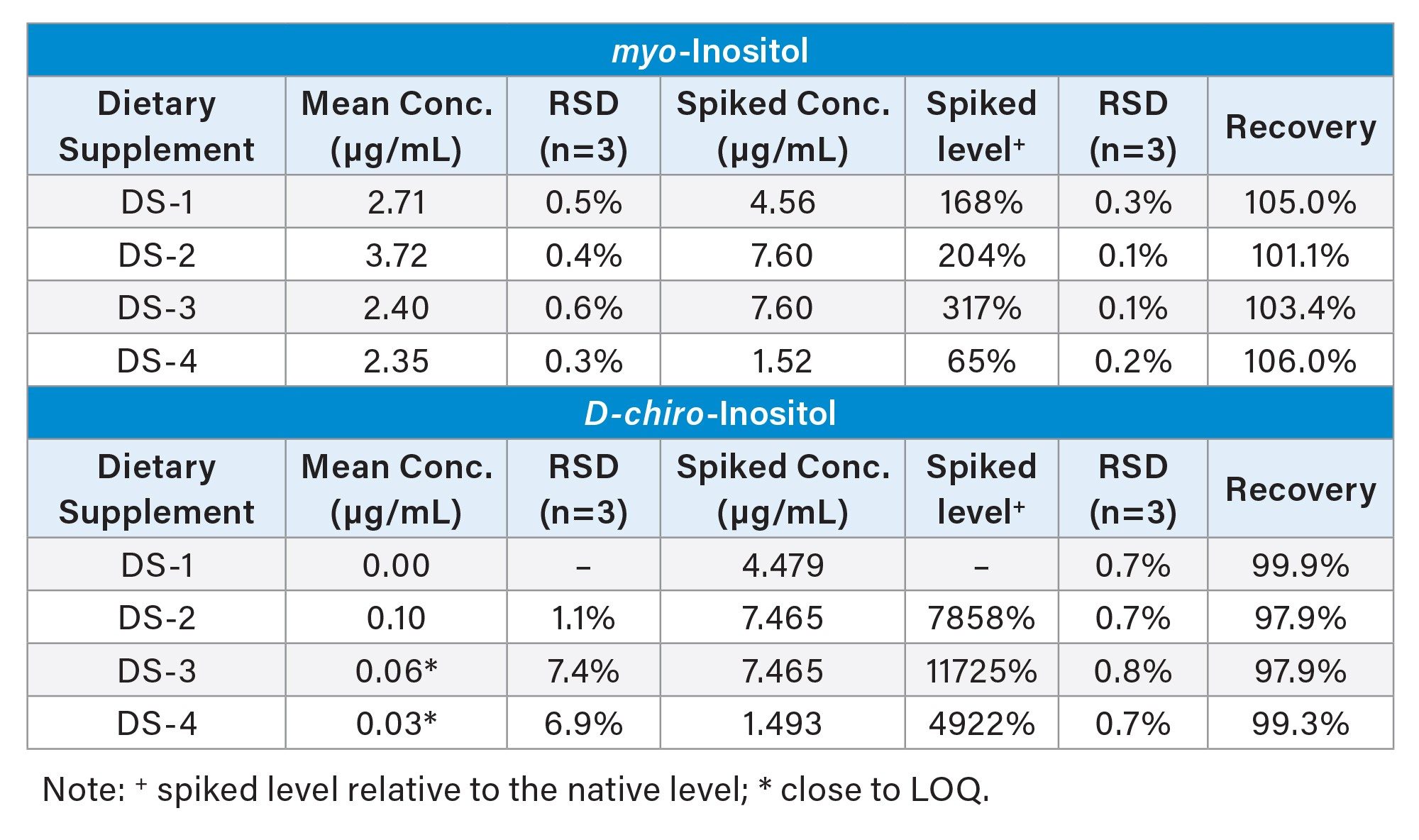
Sample Analysis Results
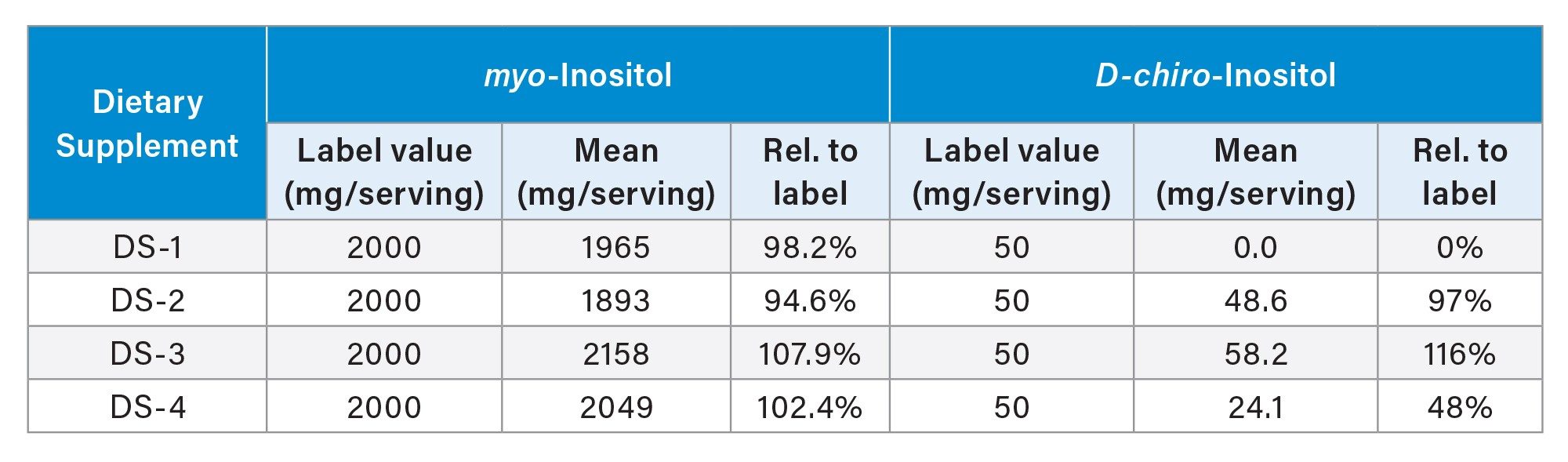
Table 3 presents the sample analysis results for four supplements and a comparison with their label-claimed values. The mean myo-inositol contents closely matched the label values, ranging from 94.6% to 107.9%, whereas the D-chiro-inositol results exhibited a markedly different picture. While DS-2 and DS-3 results matched the label values (97% and 116%, respectively), DS-1 contained no detectable D-chiro-inositol, and DS-4 had approximately half of its label value (48%). These findings underscore the importance of enhancing quality control measures in the manufacturing of D-chiro-inositol containing supplements.
Conclusion
A HILIC-MS method has been developed for the simultaneous analysis of multiple free inositol stereoisomers in dietary supplements, using an ACQUITY BEH Amide Column on an Arc Premier System coupled with an ACQUITY QDa II Mass Detector. The ACQUITY BEH Amide Column delivered excellent resolution and specificity for separating inositol stereoisomers, while the compact ACQUITY QDa II Mass Detector provided outstanding sensitivity, precision, and accuracy for quantification. Instrument control, MS data acquisition and processing were performed using Empower CDS. Overall, this method offers a robust and practical solution for routine analysis of multiple free inositol stereoisomers in dietary supplements.
References
- Monnard, I., Bénet, T., Jenni, R. et al. Plasma and Urinary Inositol Isomer Profiles Measured by UHPLC-MS/MS Reveal Differences in scyllo-Inositol Levels Between Non-Pregnant and Pregnant Women. Anal Bioanal Chem 412, 7871–7880 (2020). https://doi.org/10.1007/s00216-020-02919-8.
- DiNicolantonio JJ, H O'Keefe J. Myo- Inositol for Insulin Resistance, Metabolic Syndrome, Polycystic Ovary Syndrome and Gestational Diabetes. Open Heart 2022;9:e001989. http://doi:10.1136/openhrt-2022-001989.
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The Effectiveness of Myo-Inositol and D-chiro Inositol Treatment in Type 2 Diabetes. Int. J. Endocrinol. 2016, 2016, 1–5. http://dx.doi.org/10.1155/2016/9132052.
- Official Methods of Analysis (2019) 21st Ed., AOAC INTERNATIONAL, Rockville, MD, Method 2011.18.
- USP. Inositol. In: USP–NF. Rockville, MD: USP; May 1, 2020. DOI: https://doi.org/10.31003/USPNF_M2307_05_01.
- USP. D-chiro-Inositol. In: USP–NF. Rockville, MD: USP; Dec 1, 2020. DOI: https://doi.org/10.31003/USPNF_M10997_02_01.
- Pazourek, J. (2014). Fast Separation and Determination of free myo-Inositol by Hydrophilic Liquid Chromatography. Carbohydrate Research, 391, 55–60. doi:10.1016/j.carres.2014.03.010.
- Megías-Pérez, R., Ruiz-Matute, A. I., Corno, M., & Kuhnert, N. (2019). Analysis of Minor Low Molecular Weight Carbohydrates in Cocoa Beans by Chromatographic Techniques Coupled to Mass Spectrometry. Journal of Chromatography A, 1584, 135–143. doi:10.1016/j.chroma.2018.11.033.
720009186, December 2025