Rapid Quantification of Grape Juice Phenolics Using UPLC-QDa Mass Detection
Abstract
Phenolic compounds, abundant in grapes and grape products, act as antioxidants, combating oxidative stress in the body. We employed a rapid UPLC-MS method to quantify 16 phenolic compounds in 49 grape juice samples of varying grape varieties. Anthocyanins predominated among the compounds analyzed. Our findings validate UPLC-QDa Mass Detector as a reliable method for routine analysis of phenolic compound in grape juice.
Benefits
- Efficiently determine 16 phenolic compounds in just 4.5 minutes per sample
- Reduced solvent use by 93%, aligning with the green chemistry principle of waste reduction
- High sensitivity phenolic compounds detection with the Waters ACQUITY™ UPLC™ H-Class System paired with the Waters ACQUITY™ QDa™ Mass Detector, this method enables precise mass confirmation and accurate quantification of phenolic compounds at concentrations as low as 1.35 µg/L (e.g.: malvidin-3-O-glucoside)
Introduction
Phenolic compounds are largely present in grapes and grape-derived products, and they are important antioxidant substances that fight against oxidative stress and reduce its consequences in the human body.4,5,6 Oxidative stress is involved in many pathologies (e.g.: ischemia, atherosclerosis, Alzheimer’s disease), hence the nutraceutical value of grapes arouses interest in studying more carefully their phenolic profile.
Grape juice is the simplest product obtained from grapes and it is mainly produced from Vitis labrusca L. grapes and their hybrids such as Bordo, Concord, Isabel, Isabel Precoce (Vitis labrusca) and BRS-Magna and BRS-Violeta (hybrids of Vitis vinifera L. x Vitis labrusca).
Phenolic compounds are commonly determined through a non-specific method of quantification that expresses the results in terms of total phenolic content. Very few specific methods for the quantification of phenolic compounds in grape juice are cited in literature, although, in most cases, those methods have a long time of analysis, are not very sensitive and/or determine only a few compounds.2,4,5 Ultra-performance liquid chromatography coupled to mass spectrometry (UPLC-MS) is a promising tool for phenolic compound determination in grape juice as it allows for accurate, sensitive, and fast analysis of these compounds.
In this study, the objective was to quantitatively analyze 16 phenolic compounds present in 49 integral grape juice samples derived from various grape varieties. A rapid and validated UPLC-MS method was employed, as detailed in, to facilitate accurate measurements and robust analysis.1
Experimental
Peonidin-3,5-diglucoside, malvidin-3,5-diglucoside, malvidin-3-O-glucoside, cyanidin-3,5-diglucoside, (-)-epigallocatechin gallate, (-)-epicatechin gallate, (-)-epicatechin, procyanidin B1 and procyanidin B2 were obtained from Extrasynthese (Genay, France). Taxifolin, rutin, trans-resveratrol, quercetin, myricetin, (+)-catechin, and kaempferol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was supplied from Fluka Analytical (Munich, Germany). Methanol LC-MS and formic acid for analysis were supplied by Merck (Darmstadt, Germany).
Embrapa (Brazilian Agricultural Research Corporation – Grape and Wine Research Center) in Bento Gonçalves, Rio Grande do Sul, Brazil supplied all grape juice samples belonging to four different agronomical experiments (specific agronomical aspects of the experiments were not assessed in this study): juices from grapes cultivated in the conventional farming system, juices from grapes cultivated in the organic farming system, juices from grapes cultivated under different levels of soil fertilization and juices from grapes from different harvests.
Grape juices were prepared in an innovative system denominated Integral Juicer (developed and patented by researchers from Embrapa Grape and Wine Research Center).Peonidin-3,5-diglucoside, malvidin-3,5-diglucoside, malvidin-3-O-glucoside, cyanidin-3,5-diglucoside, (-)-epigallocatechin gallate, (-)-epicatechin gallate, (-)-epicatechin, procyanidin B1 and procyanidin B2 were obtained from Extrasynthese (Genay, France). Taxifolin, rutin, trans-resveratrol, quercetin, myricetin, (+)-catechin, and kaempferol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was supplied from Fluka Analytical (Munich, Germany). Methanol LC-MS and formic acid for analysis were supplied by Merck (Darmstadt, Germany).
Embrapa (Brazilian Agricultural Research Corporation – Grape and Wine Research Center) in Bento Gonçalves, Rio Grande do Sul, Brazil supplied all grape juice samples belonging to four different agronomical experiments (specific agronomical aspects of the experiments were not assessed in this study): juices from grapes cultivated in the conventional farming system, juices from grapes cultivated in the organic farming system, juices from grapes cultivated under different levels of soil fertilization and juices from grapes from different harvests.
Grape juices were prepared in an innovative system denominated Integral Juicer (developed and patented by researchers from Embrapa Grape and Wine Research Center).3 Grape varieties used were either traditional cultivars (Bordo, Concord, Isabel, Isabel Precoce and Niagara Rosada) or cultivars developed by Embrapa Grape and Wine’s Genetic Improvement Program (BRS-Carmem, BRS-Cora, BRS-Magna, BRS-Rubea, BRS-Violeta and Seleção 13).
Grape varieties used were either traditional cultivars (Bordo, Concord, Isabel, Isabel Precoce and Niagara Rosada) or cultivars developed by Embrapa Grape and Wine’s Genetic Improvement Program (BRS-Carmem, BRS-Cora, BRS-Magna, BRS-Rubea, BRS-Violeta and Seleção 13).
LC Parameters
|
Instrument: |
ACQUITY UPLC H-Class System |
|
Column: |
Waters™ ACQUITY UPLC BEH™ C18 Column (50 mm x 2.1 mm, 5 µm) (p/n: 186003108) with a guard column of the same material (5 mm x 2.1 mm, 5 µm) (p/n: 186007769) |
|
Mobile phase A: |
formic acid and water (2:98 v/v) |
|
Mobile phase B: |
methanol, formic acid, and water (90:2:8 v/v) |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.45 mL/min |
|
Gradient: |
Table 1 |
LC Gradient Settings
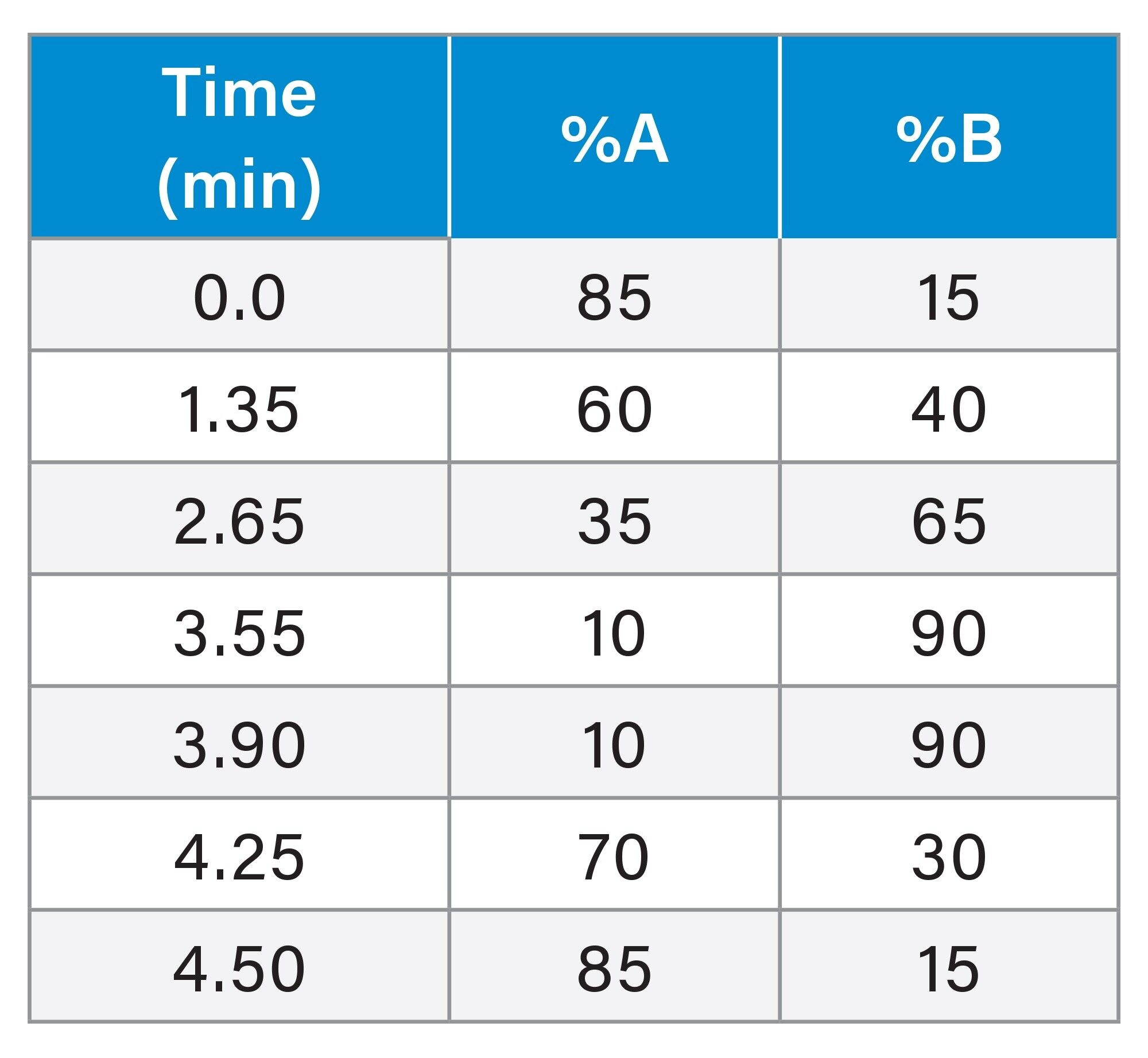
The chromatograms were acquired over a duration of 4.5 minutes using the Empower™ Software, which handled system control, data acquisition, and processing. Following each injection, the column was then re-equilibrated with the initial mobile phase condition (15% B) for three minutes.
MS Parameters
|
Instrument: |
ACQUITY QDa Mass Detector* |
|
Acquisition mode: |
Selected Ion Recording (SIR) |
|
Capillary voltage: |
-0.8 kV (ESI-) and +1.5 kV (ESI+) |
|
Probe temperature: |
600 °C |
*equivalent or better performance is achieved on ACQUITY QDa II Mass Detector
Individual mass to charge (m/z) for target analytes used in the SIR method, ionization mode and specific cone voltage for each compound are shown in Table 2.
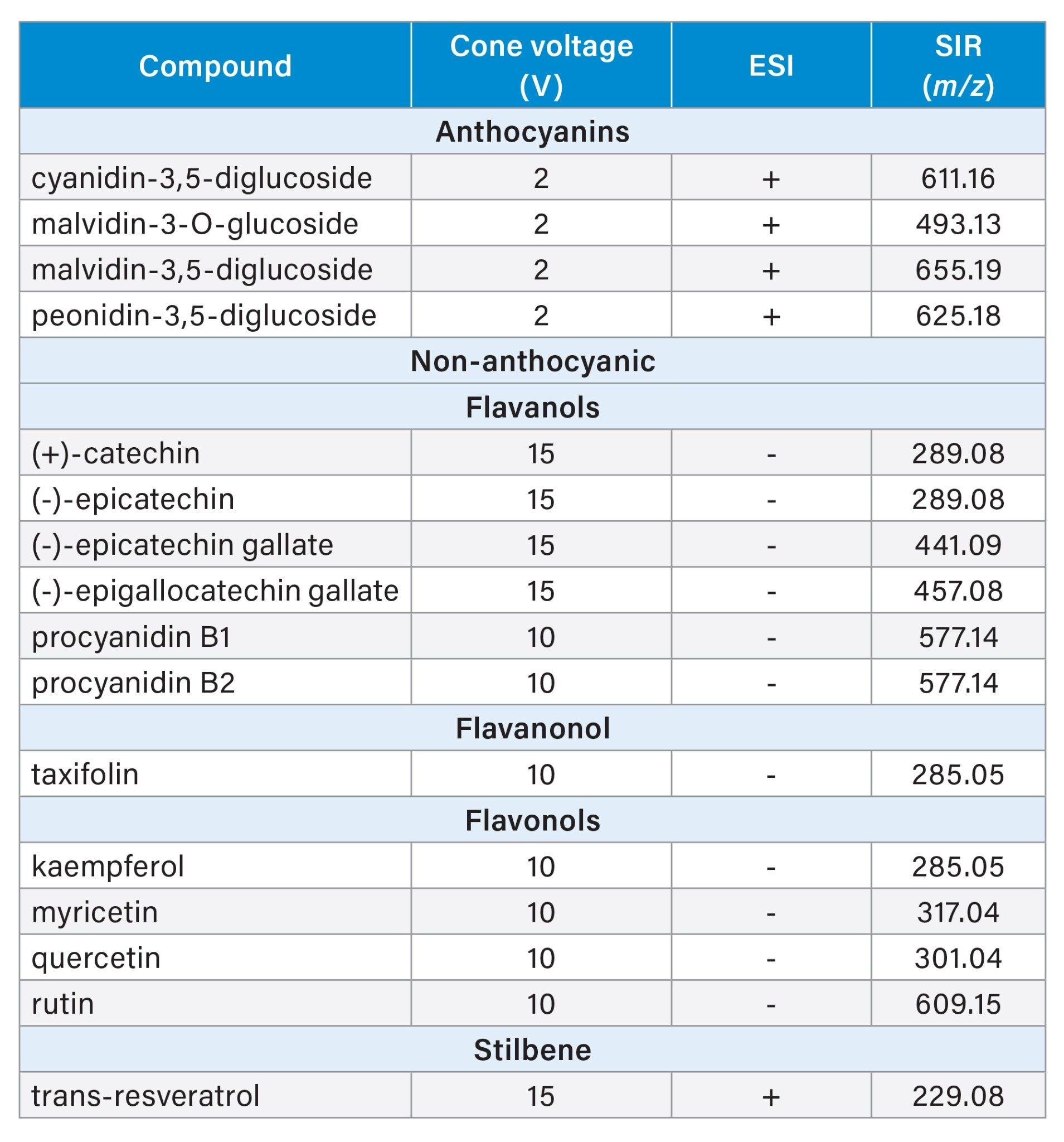
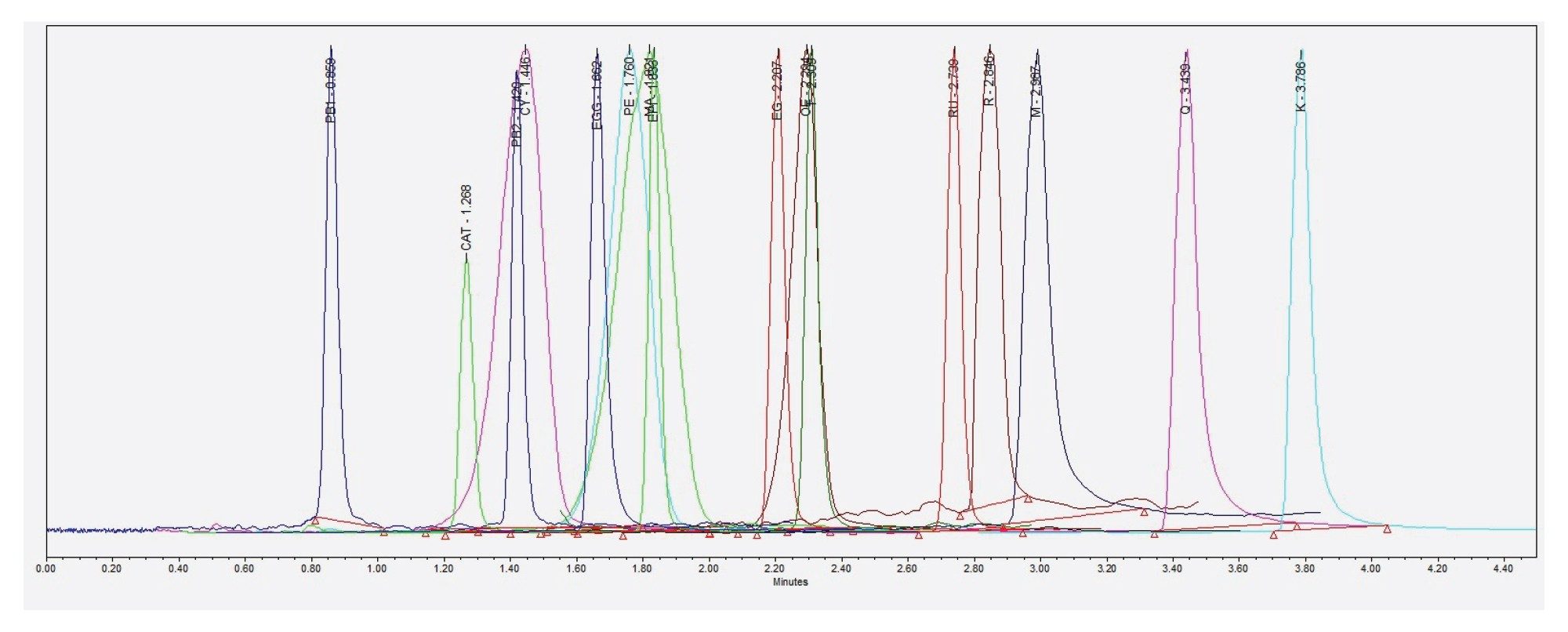
Fresh working standard solutions were prepared from intermediate solutions in three groups: anthocyanins, procyanidins, and other non-anthocyanic compounds. These were diluted to the midpoint of the calibration curve using a solution of formic acid, water, and methanol (2:84.5:13.5 v/v). Before use, the solutions were centrifuged at 10,000 rpm for 10 minutes, and the supernatant was injected. Chromatograms for the 16 phenolic compounds studied are shown in Figure 1.
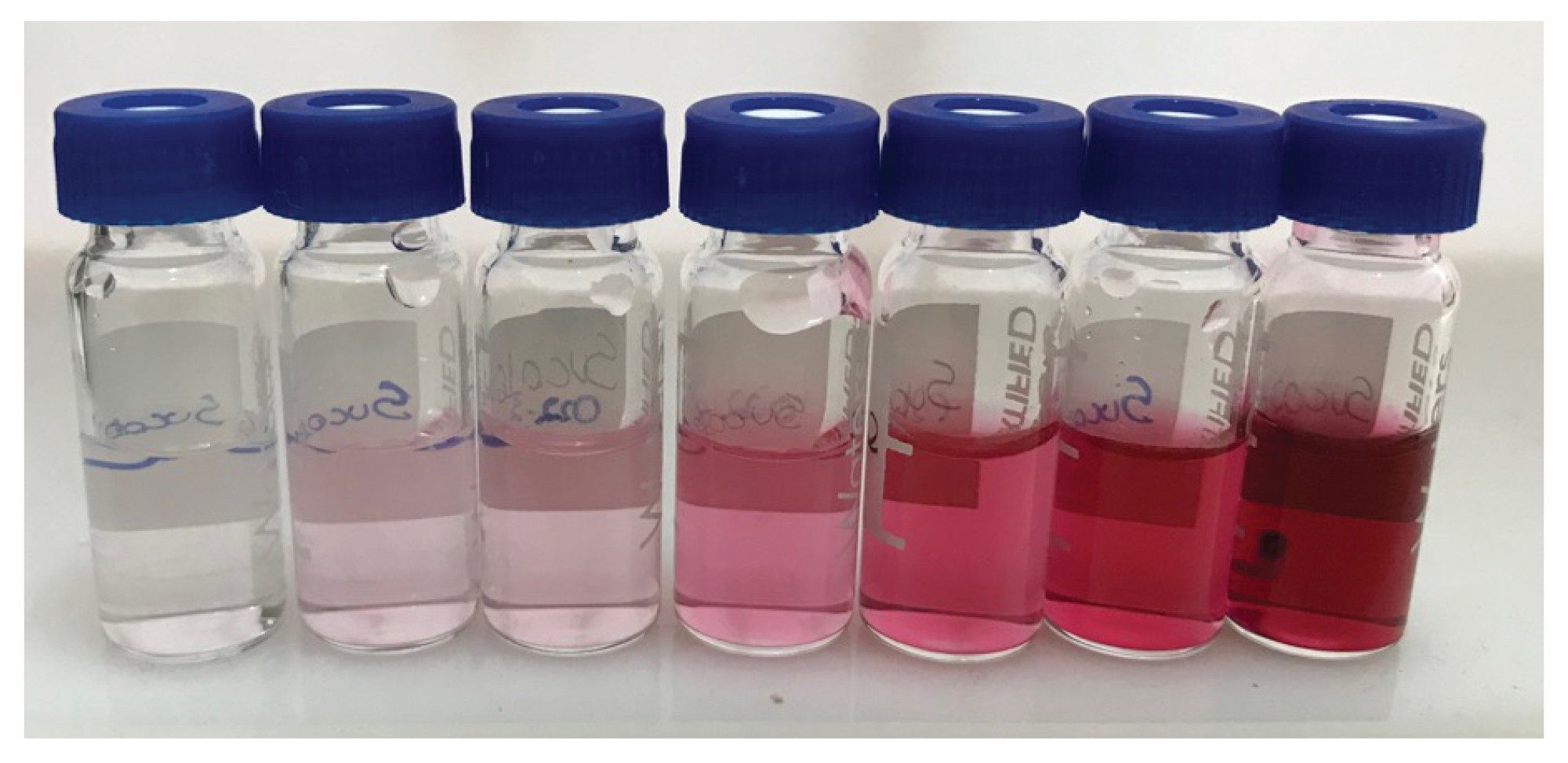
For sample preparation, grape juice samples were centrifuged similarly, the supernatant was diluted at least fivefold (to fit within the calibration range) with the same solution, and then centrifuged again. The supernatant was injected. Figure 2 illustrates an example of grape juice dilution.
Results and Discussion
Few methods exist for quantifying phenolic compounds in grape juice, and those that do are often insensitive, time-consuming, and limited in scope. Chromatographic techniques like UPLC have advanced sensitivity and reduced analysis time, enabling the detection of low-concentration molecules while minimizing solvent use and waste. Mass detection, highly selective and effective in complex matrices like grape juice, complements UPLC. Table 3 presents the quantitative results of phenolic compounds in 49 grape juice samples analyzed by UPLC-MS.
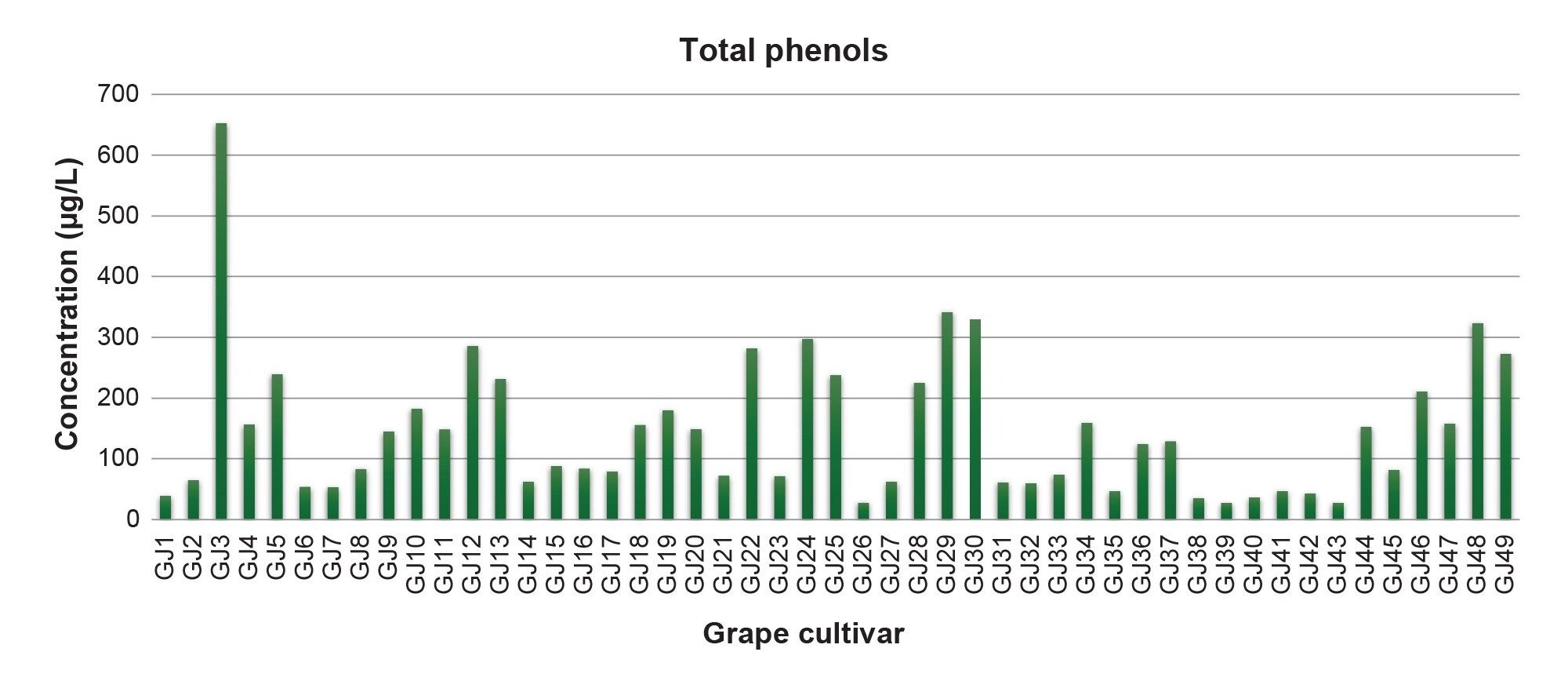
Figure 3 presents total phenols concentrations of the 49 grape juice samples studied.
Organic juices were made with Bordo, BRS-Cora, BRS-Carmem, Concord, Isabel Precoce, BRS-Magna, BRS-Rubea, BRS-Violeta, and Seleção 13 (an experimental variety). Conventional juices used Bordo, BRS-Cora, BRS-Carmem, Concord, Isabel, Isabel Precoce, BRS-Magna, BRS-Rubea, and BRS-Violeta. Isabel was exclusive to conventional juices, and Seleção 13 was exclusive to organic juices, while other varieties were common to both. Organic juices had higher phenolic compounds in most varieties: Bordo, BRS-Carmem, BRS-Cora, BRS-Magna, BRS-Rubea, Concord, and Isabel Precoce. Seleção 13 had the highest phenolic content among organics, particularly malvidin-3,5-diglucoside and peonidin-3,5-diglucoside. However, conventional BRS-Violeta had the highest phenolic concentration overall. BRS-Cora had the lowest phenolic content in both groups.
Bordo and BRS-Magna juices from different harvests (2017, 2018, and 2019) were studied. Samples were prepared at each harvest and analyzed simultaneously, revealing that older juices had fewer phenolic compounds, except for myricetin, which increased with age. Bordo juices consistently had higher anthocyanin levels, while BRS-Magna juices were richer in non-anthocyanic compounds.
The impact of soil fertilization on phenolic content was examined using Niagara Rosada juices. Grapes grown with intermediate fertilization produced juices with the highest phenolic levels.
Across the 49 grape juice samples, anthocyanins were the most abundant phenolics in most of them, with malvidin-3,5-diglucoside being the predominant compound, especially in BRS-Violeta conventional juice. Peonidin-3,5-diglucoside was the second most abundant anthocyanin. Flavanols were the second most prevalent phenolics, with (+)-catechin most abundant in Isabel Precoce organic juice. Procyanidin B1 was more prevalent than Procyanidin B2, and myricetin was the most abundant flavonol, especially in BRS-Violeta conventional juice. Quercetin appeared in more samples than myricetin but in smaller quantities. Taxifolin was only found in Isabel Precoce juices, and trans-resveratrol was most abundant in organic Isabel Precoce juice. These findings align with previous grape juice studies.2,4,5
Conclusion
The ACQUITY UPLC System coupled with the ACQUITY QDa Mass Detector provided several key benefits for the analysis of phenolic compounds in grape juice:
- Simultaneous detection and quantification of 16 phenolic compounds at µg/L levels.
- Simplified sample processing by eliminated the need for complex sample preparation or prior purification of the grape juice, streamlining the analytical workflow.
- Mass identification of target compounds, revealed that anthocyanins were the most abundant phenolic compounds in the samples analyzed.
- High phenolic content in specific varieties such as Bordo, BRS-Violeta, and Seleção 13 grape juices, highlighting their potential health benefits and quality.
Acknowledgement
We would like to acknowledge the contributions of Celito Crivellaro Guerra, Letícia Flores da Silva, Luísa Carolina Wetzstein, Carlos Henrique Junges, Marco Flôres Ferrão, and Ana Maria Bergold from their respective institutions: the Programa de Pós-Graduação em Ciências Farmacêuticas, Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul; LACEM – Laboratório de Cromatografia e Espectrometria de Massas, Embrapa Uva e Vinho; Tecnologia em Viticultura e Enologia, Instituto Federal do Rio Grande do Sul; and the Programa de Pós-Graduação em Química, Instituto de Química, Universidade Federal do Rio Grande do Sul.
References
- CANEDO-REIS, N.A.P.; GUERRA, C.C.; DA SILVA, L.F.; WETZSTEIN, L. C.; JUNGES, C.H.; FERRÃO, M.F.; BERGOLD, A.M. Fast Quantitative Determination of Phenolic Compounds in Grape Juice by UPLC-MS: Method Validation and Characterization of Juices Produced With Different Grape Varieties. Journal of Food Measurement and Characterization, v. 15, p. 1044–1056, 2021.
- DANI, C.; OLIBONI, L. S.; VANDERLINDE, R.; PRA, D.; DIAS, J. F.; YONEAMA, M. L.; BONATTO, D.; SALVADOR, M.; HENRIQUES, J. A. P. Antioxidant Activity and Phenolic and Mineral Content of Rose Grape Juice. Journal of Medicinal Food, [s. l.], v. 12, n. 1, p. 188–192, 2009.
- GUERRA, C. C.; BITARELO, H.; BEN, R. L.; MARIN, A. Sistema Para Elaboração de suco de uva integral em pequenos volumes: Suquificador Integral. Documentos, 96. 1. ed. Bento Gonçalves, RS: Embrapa Uva e Vinho, 2016.
- NATIVIDADE, M. M. P.; CORRÊA, L. C.; SOUZA, S. V. C. De; PEREIRA, G. E.; LIMA, L. C. de O. Simultaneous Analysis of 25 Phenolic Compounds in Grape Juice for HPLC: Method Validation and Characterization of São Francisco Valley Samples. Microchemical Journal, [s. l.], v. 110, p. 665–674, 2013.
- PADILHA, C. V. da S.; MISKINIS, G. A.; DE SOUZA, M. E. A. O.; PEREIRA, G. E.; DE OLIVEIRA, D.; BORDIGNON-LUIZ, M. T.; LIMA, M. dos S. Rapid Determination of Flavonoids and Phenolic Acids in Grape Juices and Wines by RP-HPLC/DAD: Method Validation and Characterization of Commercial Products of the New Brazilian Varieties of Grape. Food Chemistry, [s. l.], v. 228, p. 106–115, 2017.
- SILVA, L. F. Da; GUERRA, C. C.; KLEIN, D.; BERGOLD, A. M. Solid Cation Exchange Phase to Remove Interfering Anthocyanins in the Analysis of other Bioactive Phenols in Red Wine. Food Chemistry, [s. l.], v. 227, p. 158–165, 2017.
720008376, June 2024