Analysis of Azithromycin on the Alliance™ iS HPLC System: System Performance under Challenging Method Conditions
본 응용 개요서는 구체적인 실험 내용을 포함하지 않습니다.
Abstract
Many existing reversed-phase monographs contain a combination of conditions that can impact long term system performance. For example, traditional HPLC gradient methods may use both buffered and organic mobile phases at proportions that the salt may be insoluble, potentially leading to precipitation of the buffer during the course of the analysis. While these methods have been validated and can be performed on a number of different HPLC systems, these conditions can lead to system-to-system variability while still meeting the relevant suitability requirements listed by the USP. The USP monograph for Azithromycin organic impurities is one such example. This application note will discuss the challenges of this method and compare the results obtained across a number of different HPLC systems.
Benefits
- Alliance iS HPLC System meets system suitability criteria for USP monograph of Azithromycin
- Superior retention time precision for high salt mobile phases under HPLC conditions
- Stable performance over 30 hours under high salt mobile phase conditions
Introduction
Many regulated legacy monographs require buffers in the mobile phase to ensure reliable performance of the method. For these traditional gradient reversed-phase applications the mobile phase transitions from a buffered, aqueous mobile phase to an organic mobile phase. The proportions of each mobile phase during elution may cause the salt to become insoluble in the water/organic composition, resulting in potential precipitation of the salt. Furthermore, under these conditions there may be variability across different HPLC systems due to design differences. For this application note, the USP monograph for Azithromycin Organic Impurities was chosen as a representative method for these conditions. This specific monograph mobile phases include a weak aqueous mobile phase buffer at a concentration of 12 mM, and a strong mobile phase with acetonitrile/methanol blend. The separation requires a gradient which has a strong elution of 25/75 coupled with a 75% organic mobile phase composition. This monograph was executed on multiple HPLC systems, using replicate injections of standards to investigate system suitability and performance differences across systems.
Experimental
Sample Description
Azithromycin (p/n: 1A00860), Azithromycin Related Compound F (p/n: 1046089), and Desosaminylazithromycin (p/n: 1046078) were obtained from the U.S. Pharmacopeia (Rockville, MD). An expired Azithromycin standard from the U.S. Pharmacopeia was used to act as a surrogate sample. All solutions were prepared in a diluent of 7:6:7 methanol: acetonitrile: and 1.73 mg/mL monobasic ammonium phosphate (pH 10.0 ± 0.05). Azithromycin standard solution was prepared at 86 µg/mL and the system suitability solution was prepared at 16.5 µg/mL and 27.0 µg/mL of Azithromycin Related Compound F and Desosaminylazithromycin, respectively. The Azithromycin sample solution was prepared at 8.6 mg/mL. All samples were prepared as described in the monograph.
LC Conditions
|
LC system: |
Alliance iS HPLC System Vendor X HPLC System Vendor Y HPLC System Vendor Z HPLC System |
|
Detection: |
UV for all systems (210 nm) |
|
Column: |
XBridge™ C18, 5µm, 4.6 x 250 mm (p/n: 186003117) |
|
Column temperature: |
60 °C |
|
Sample temperature: |
5 °C |
|
Injection volume: |
50 µL |
|
Flow rate: |
1.00 mL/min |
|
Mobile phase A: |
1.8 mg/mL anhydrous dibasic sodium phosphate (pH 8.9) |
|
Mobile phase B: |
3:1 Acetonitrile/Methanol |
|
Seal wash: |
90:10 Water/Acetonitrile (where applicable) |
|
Needle wash: |
50:50 Water/Mobile Phase B |
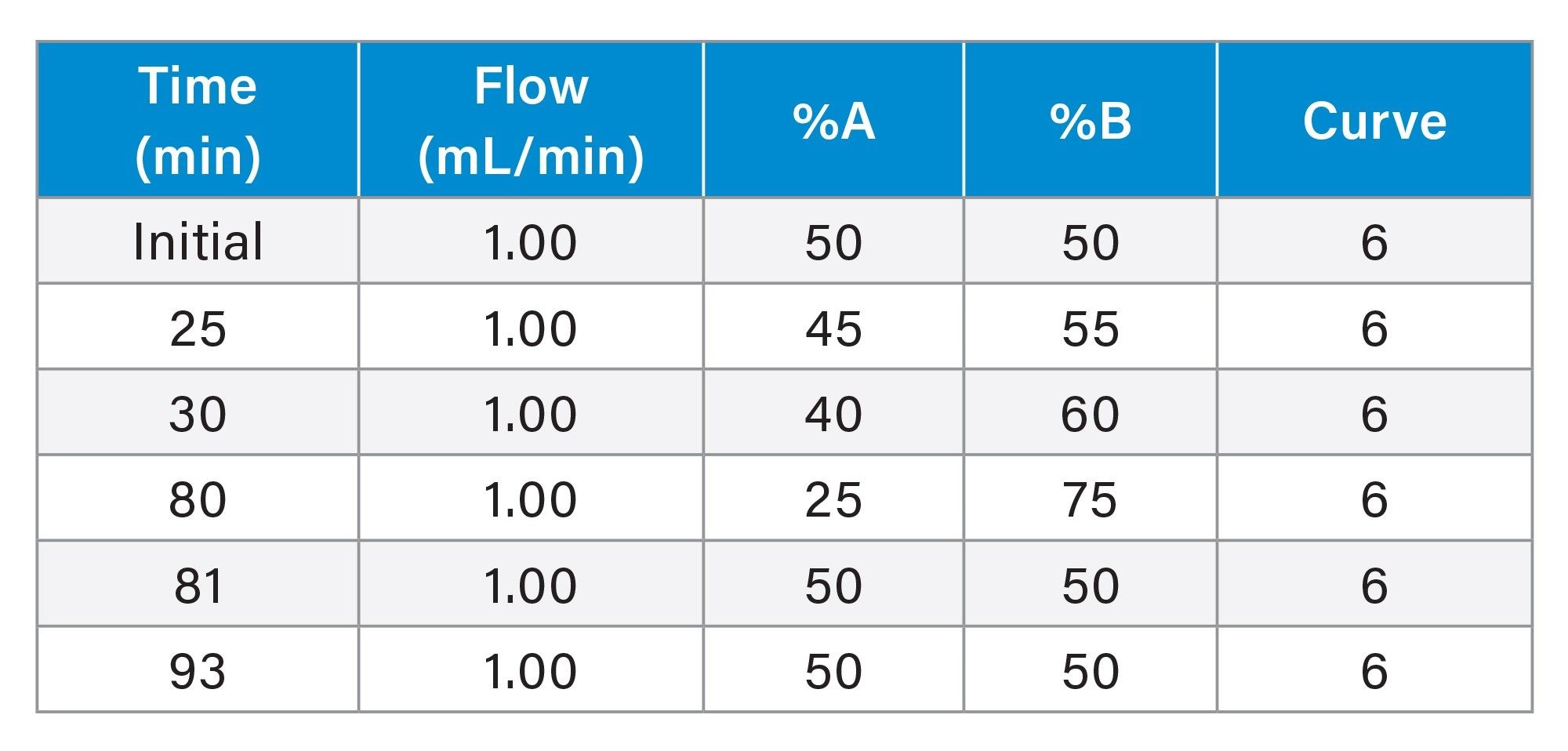
Gradient Table
Data Management
|
Chromatography software: |
Empower™ 3.7.0 |
Results and Discussion
Analysis on Alliance iS HPLC System
For this study, a prescribed analysis and process was used for consistent data quality, including the appropriate number of injections to meet systems suitability. The total run time for the 20 injections was over 31 hours. Each system was thoroughly equilibrated and showed stable system pressure before sample set submission. Data was analyzed and evaluated based on the system suitability requirements including: system suitability standard- azithromycin related compound F/desosaminylzithromycin peak to valley ratio not less than (NLT 1.4) and standard -azithromycin USP Tailing 0.8–1.5. To control system-to-system variability the same HPLC column was used on all systems tested.
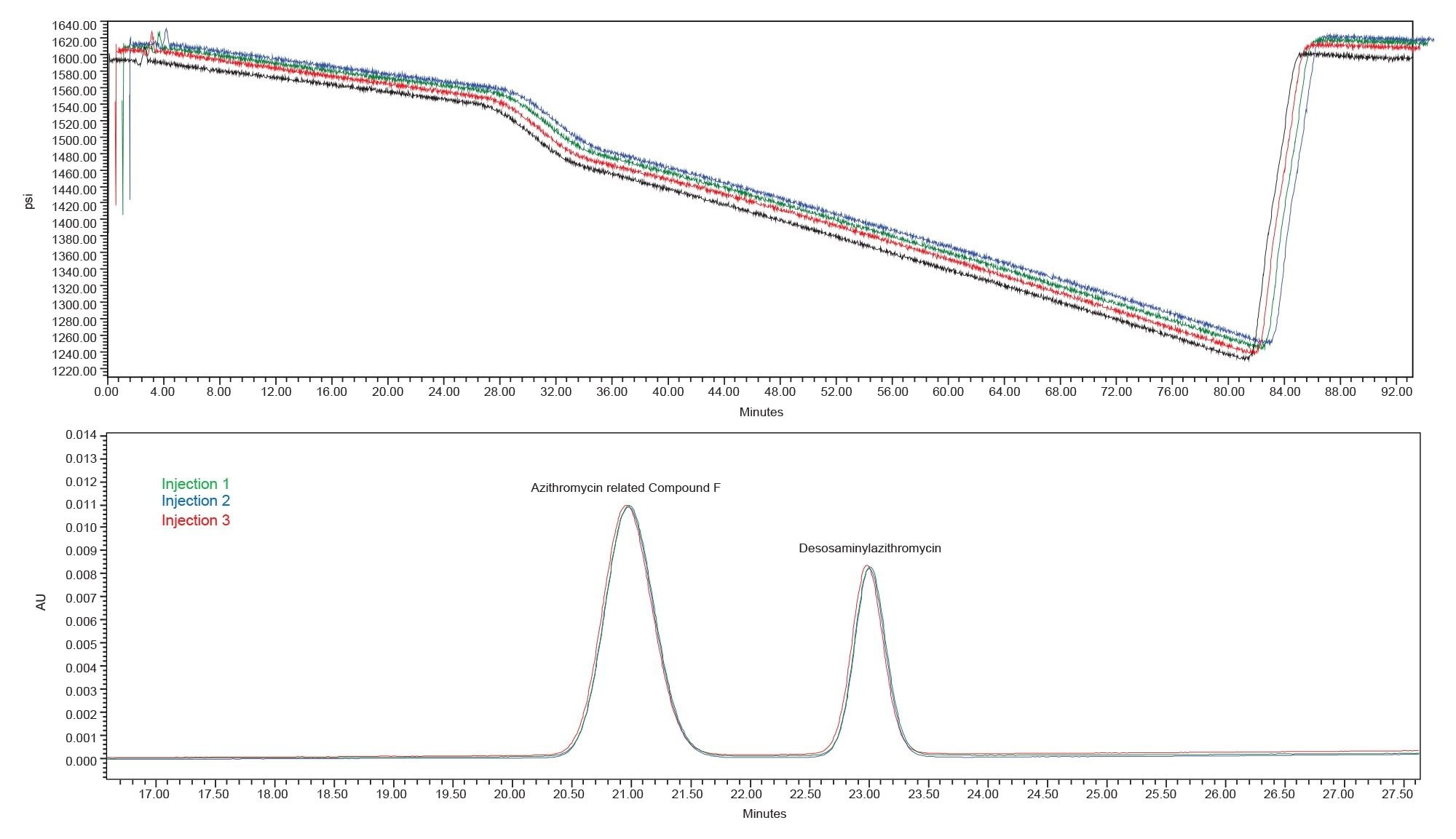
The Alliance iS was able to execute the sample set described above, running for more than 31 hours continuously. Evaluation of the system suitability results (Table 1) showed the USP tailing of azithromycin standard met the criteria. For the system suitability sample, the peak to valley ratio for azithromycin related compound F and desosaminylazithromycin could not be calculated, as the two peaks were baseline resolved with USP resolution of approximately 3.0 (Figure 1). Since peak to valley calculations are only determined for those peaks that are not baseline resolved or co-elute, the system suitability value could not be determined. When looking at non-system suitability requirements, including retention time precision, the Alliance iS HPLC System showed %RSD of less than 0.2% for all analytes for the six replicate injections over an analysis time of nine hours.
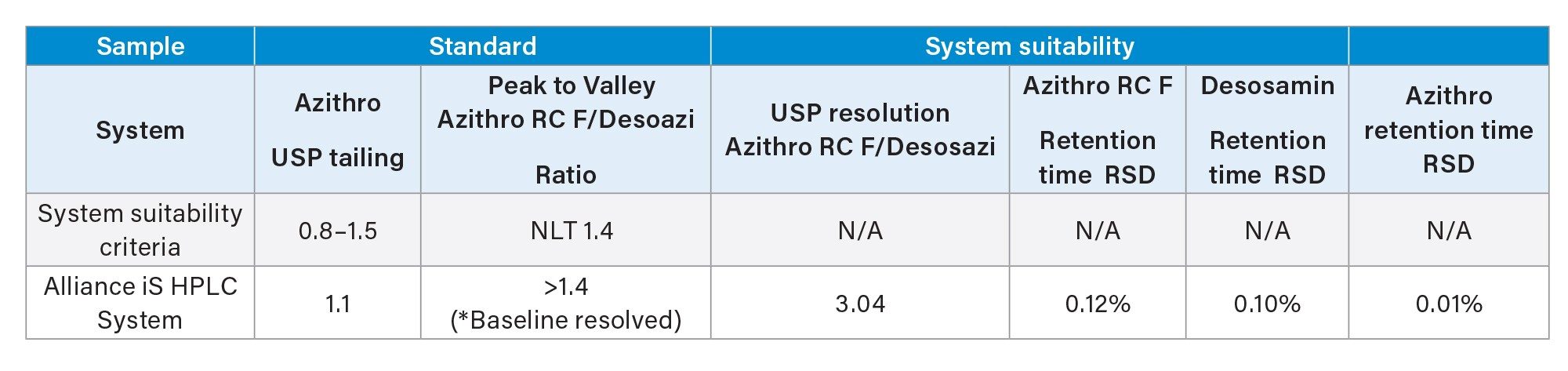
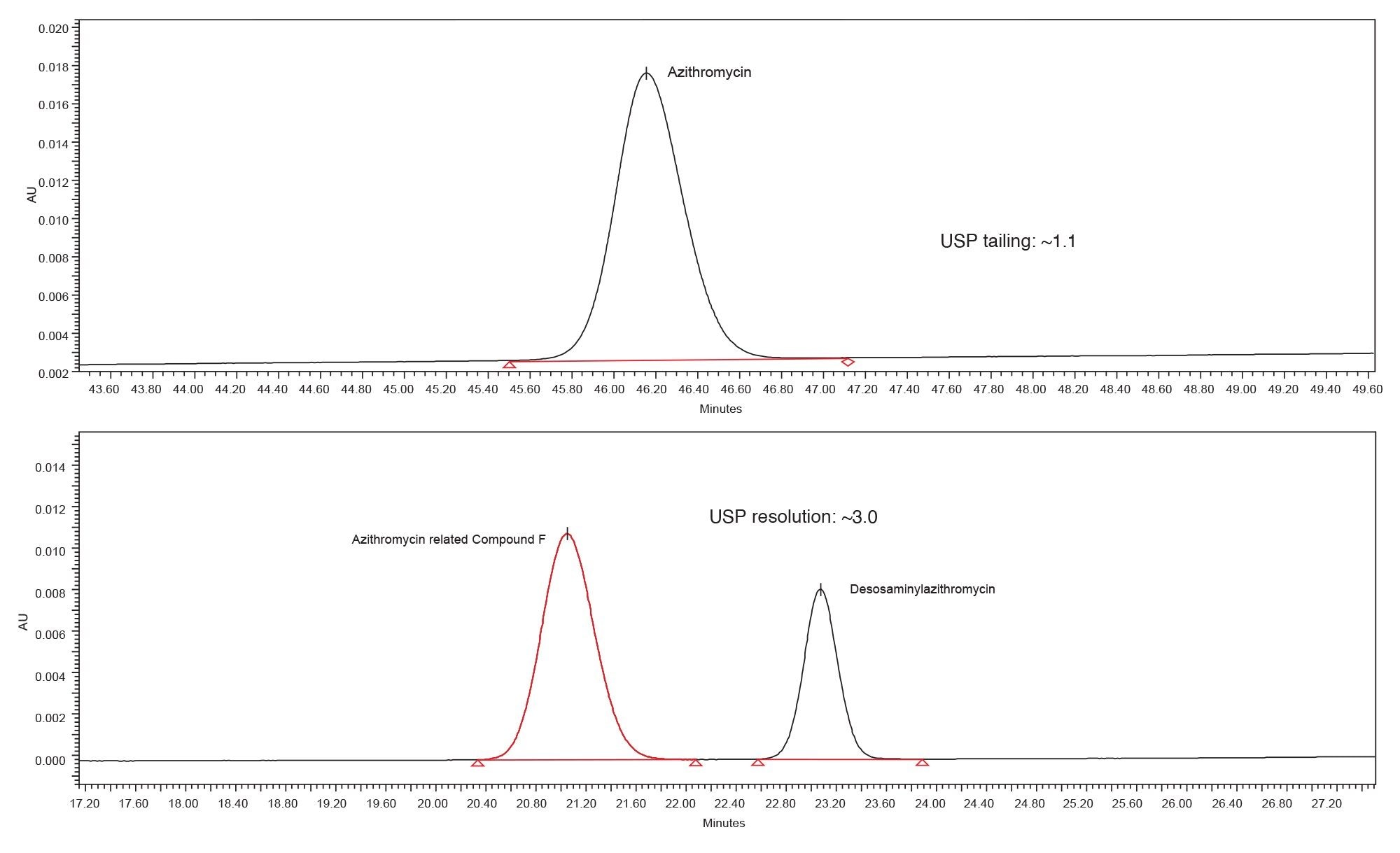
Figure 1. Representative chromatograms of the azithromycin standard solution and system suitability solution using the Alliance iS HPLC.
When analyzing the surrogate azithromycin sample, impurity peaks were detected. Per the USP monograph, the following impurity peaks are discarded: all peaks eluting before azithromycin N-oxide and after 3-deoxyazithromycin (azithromycin B) and peaks with a response less than 0.1 times the response of the azithromycin peak in the Standard solution (0.1%). Based on these criteria, one particular peak of interest met the monograph criteria for integration. After calculating the relative retention time for this peak (0.53 compared to 0.54 monograph value) and overlaying the sample chromatogram with the system suitability standard, the impurity was shown to likely be desosaminylazithromycin.
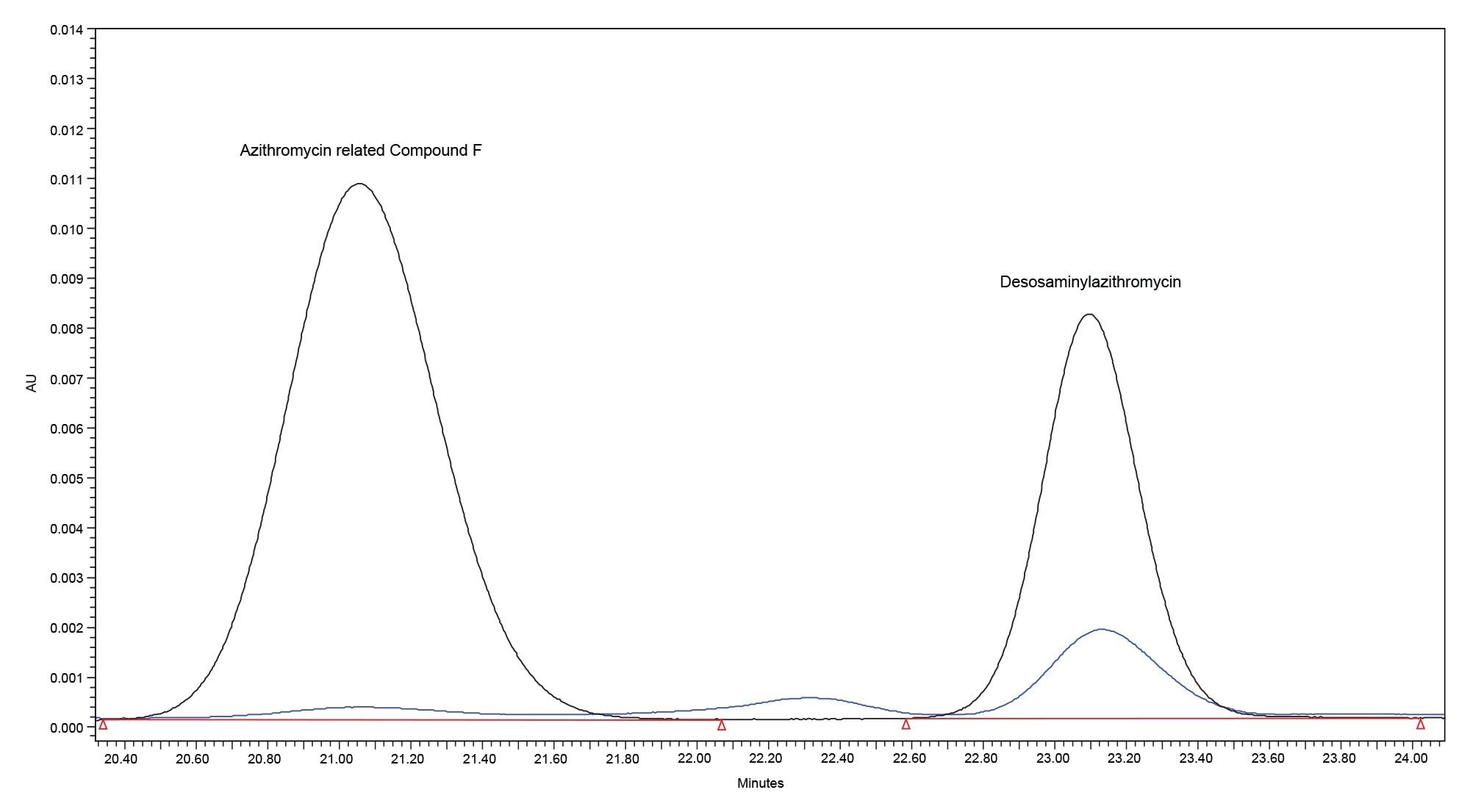
Comparison of Performance Across Different HPLC Systems
The monograph was also tested across different HPLC systems to assess the impact of system design on method performance. For the other systems tested, the analysis, as previously described, ran to completion. In general, all systems were able to to meet the system suitability criteria. Using the same column on all systems, each system produced comparable USP tailing values for azithromycin in the standard solution. In addition, all systems met the requirement for peak-to-valley of NLT 1.4 between azithromycin related compound F and desosaminylazithromycin.
Given specific characteristics of the method, it was also used to assess retention time precision under challenging conditions. Specifically, during the segment in which the API and some related impurities elute (0–25 min), the mobile phase composition changes by 0.5% per minute, which can result in retention time variability. These retention time shifts become more pronounced the later a compound elutes in the chromatogram. In addition, mobile phase compositions and gradient have the potential to cause buffer precipitation. Specifically mobile phase A consists of a 1.8 mg/mL phosphate buffer while mobile phase B contains a 3:1 mixture of Acetonitrile/Methanol with a gradient composition of up to 75% by the end of the gradient. This high organic solvent composition coupled with phosphate buffer creates conditions that may cause precipitation of the buffer salt. The precipitation of salt could impact the system in multiple ways: over pressurizing due to clogging of the system or column, or loss of system pressure/prime due to particulates in the pump. The latter may be temporary but can lead to inconsistent retention times from injection-to-injection. Because impurities are identified solely based on retention times, it is critical to have good retention time precise as variations in retention time could lead to the misidentification of impurities in the sample since many of the known impurities closely elute.
When comparing the retention time precision, for the analysis retention time standard deviation of critical compounds, the Alliance iS showed lower %RSD values for all three compounds when compared to the three other HPLC systems. Of the other systems tested, System Z HPLC system showed significantly more retention time variability for all compounds (Figure 3).
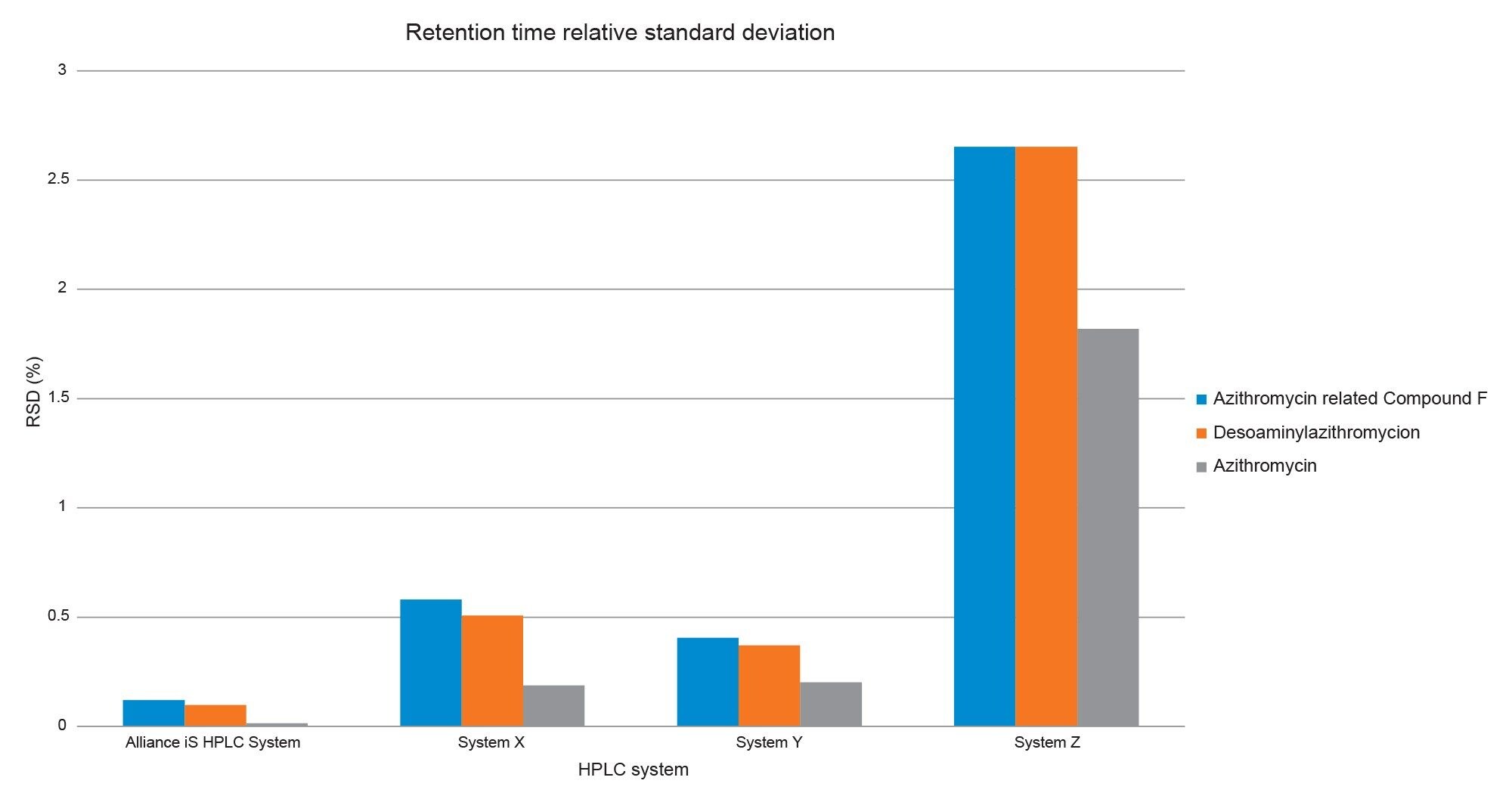
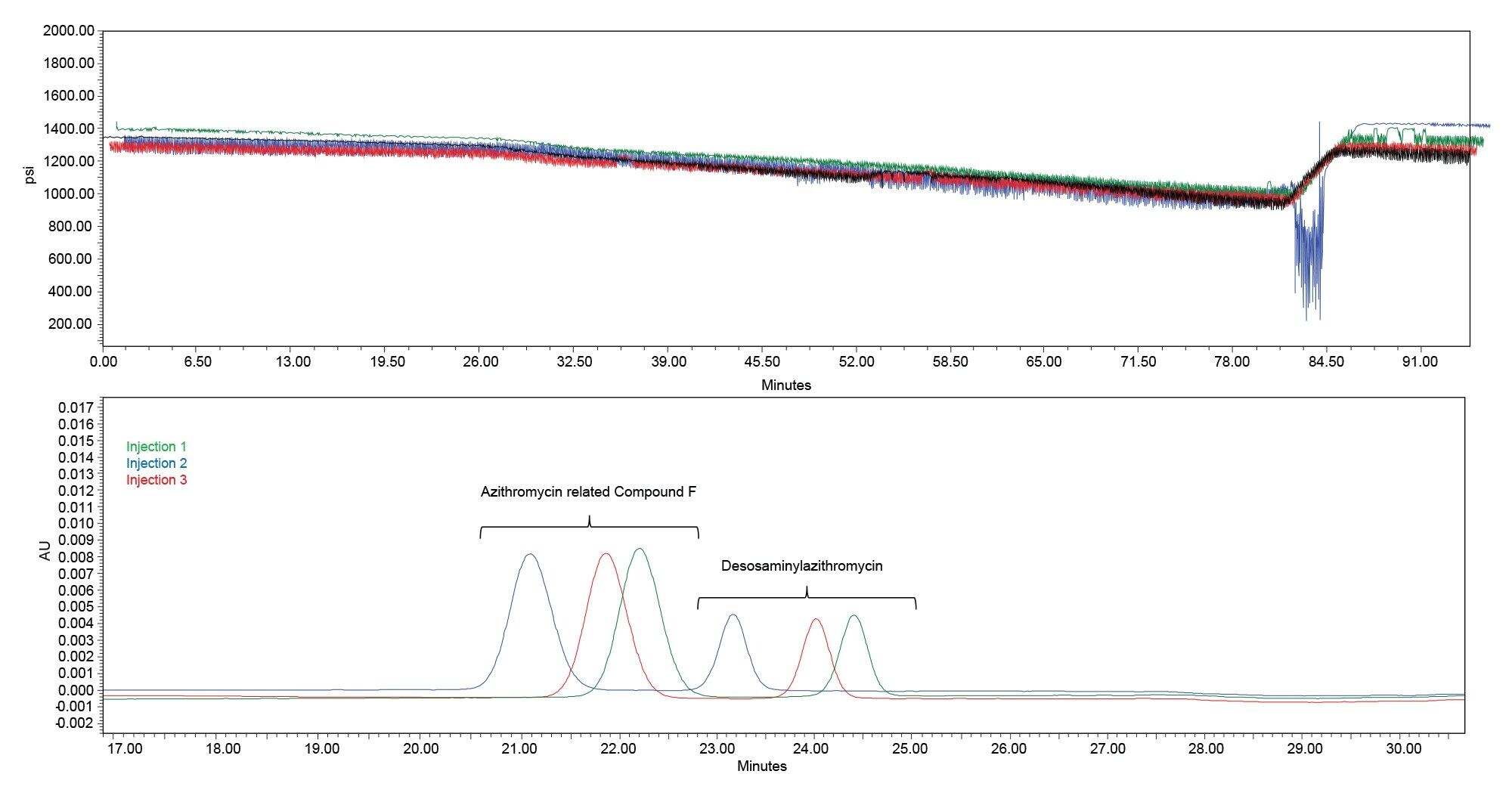
Upon closer inspection of the data, some abnormalities in system pressure were observed on a few HPLC systems. While each system was able to complete the sample set and meet the system suitability requirements there was greater retention variability across some systems. The variability varied from system to system. For system Z HPLC, system pressure oscillations were observed in the first injection (black), with increasing variability at higher composition of organic solvent. After these pressure oscillations were observed in the first injection, they continued throughout the sample set. While this system was able to complete the sample set and met both system suitability requirements, the higher retention time %RSD values were observed (>2%). Below we see the overlaid system pressure channels of the first four injections on System Z HPLC System. Data is offset for comparative purposes.
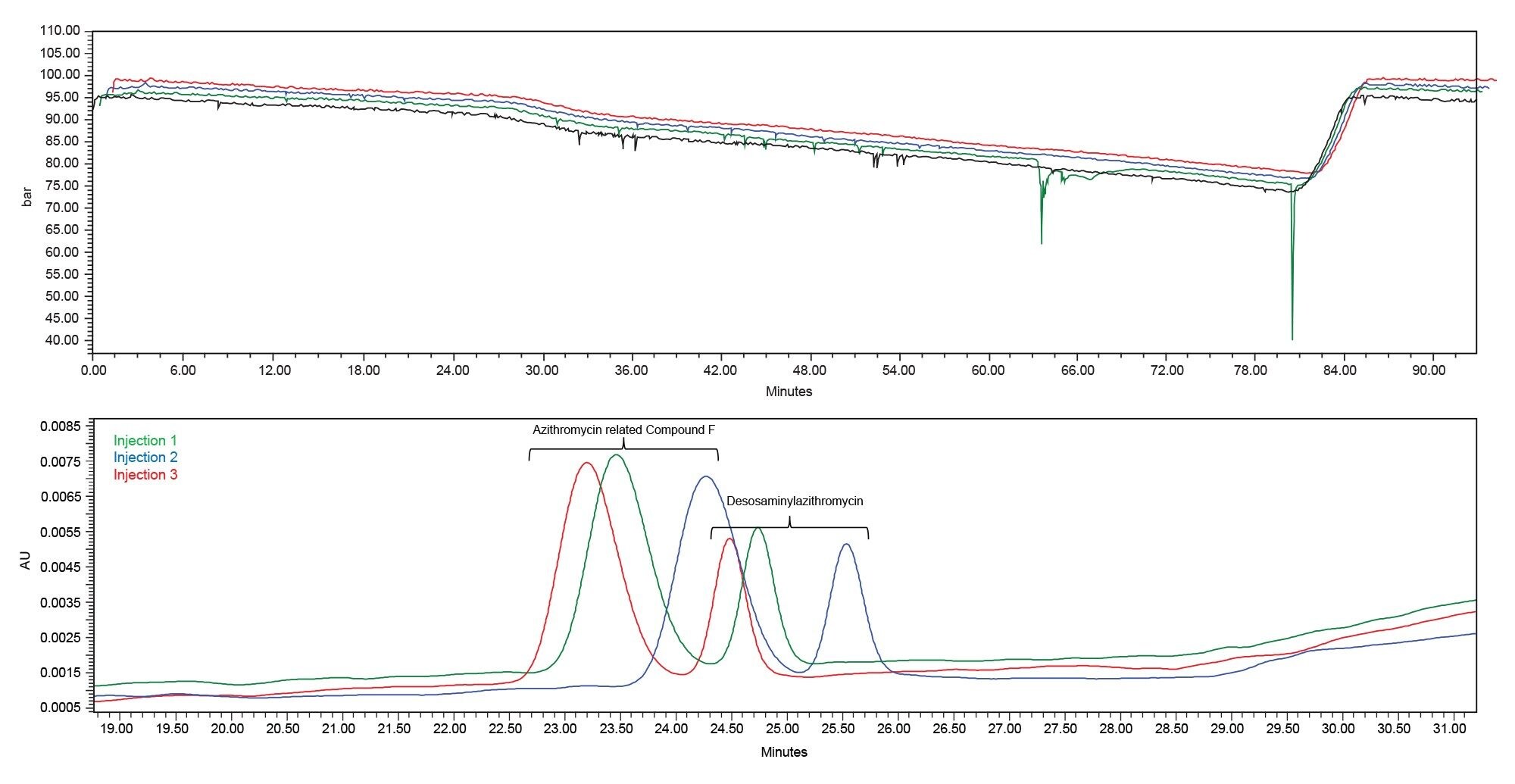
Another HPLC system tested produced some variability with all %RSD’s between 0.2–0.6%. For the first four injections of the sample set from System X, there were minor drop offs in pressure (Figure 5). During the first injection (black) the system pressure fluctuations were typical for gradient analysis up until approximately 30 minutes, at which point some minor pressure dips were observed. Subsequent injections (green, blue, red) produced larger pressure dips. While this system was able to complete the sample set and met both system suitability requirements, the retention time variability may be a result of these momentary drops in pressure.
In comparison, the Alliance iS showed stable system pressure and retention time precision of less than 0.2% throughout the sample set. Specifically, the Alliance iS produced similar system pressure profiles throughout the sample set with no unusual dips during the gradient (Figure 5). The system also produced good retention time precision over the sample set with low %RSD’s <0.2%.
Impact of Retention Time Variability of Relative Retention Times
For impurity methods, retention time reproducibility is critical for confirmation or identification of impurities. Given there can be retention time shifts across systems due to gradient delay volumes, many monographs provide RRT for impurities as guidance to assist in identification or confirmation of impurities. While there may be shifts from system-to-system, it is typically expected that RRT would be reproducible from injection-to-injection.
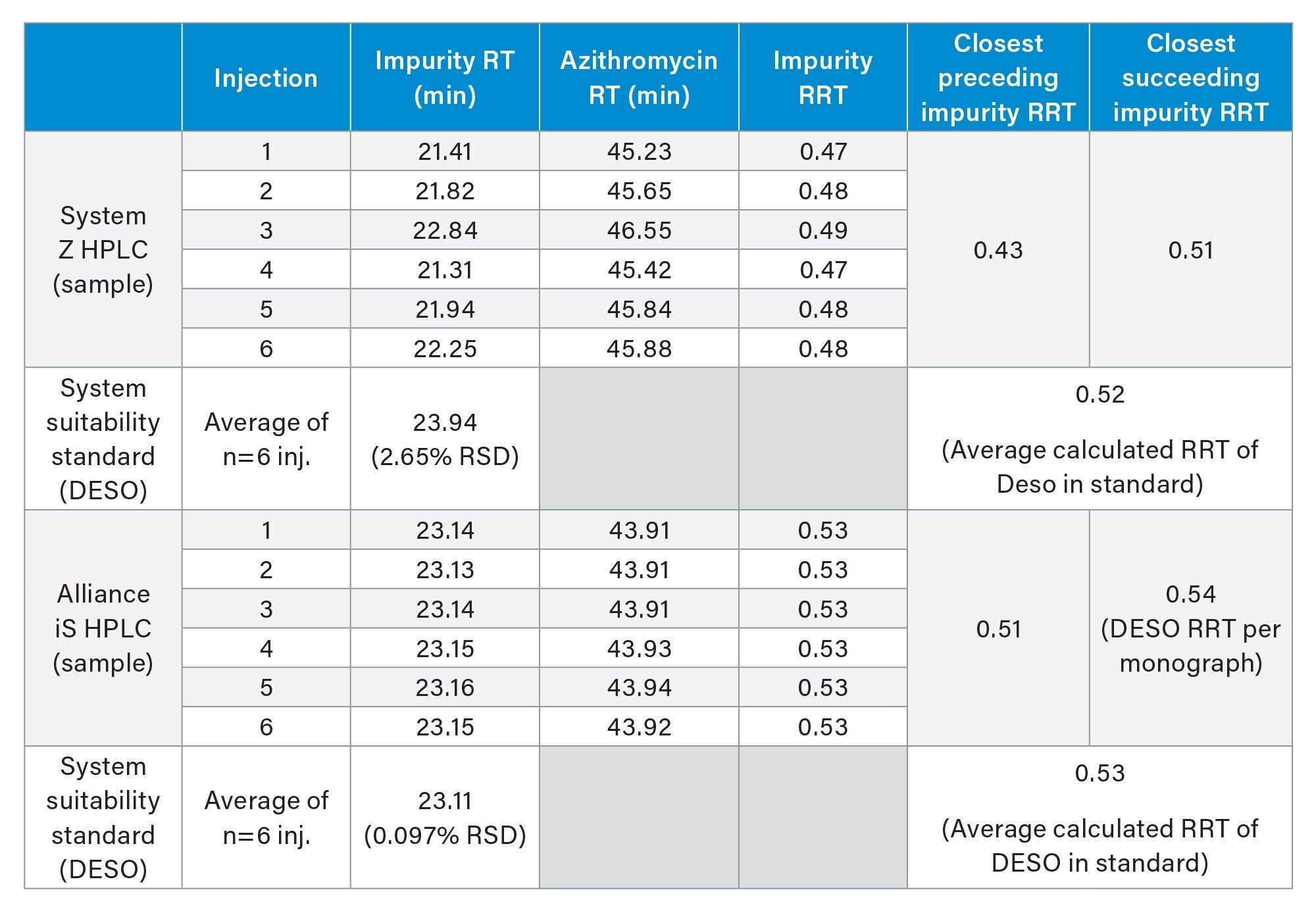
Evaluation of the RRT for the impurity peak showed the impact of retention time variability. The table below summarizes the RRT values for an impurity peak in the azithromycin sample on system Z HPLC and the Alliance iS HPLC over six replicate injections (Table 2). For system Z, the values range from 0.47 to 0.49. While these differences may seem small, there are two impurities specified in the monograph with similar RRT’s, and the unknown peak on System Z shows RRTs in between both. Thus, depending on the injection, the peak may be assigned to a different impurity.
Comparatively, the Alliance iS HPLC showed a consistent RRT value across all injections. More interestingly, this impurity would be incorrectly identified using system Z after closer inspection. Because the system suitability standard contains the compound desosaminylazithromycin (DESO), the overlay of the system suitability standard and sample can provide confirmation of this peak. For the Alliance iS the peak at 23.1 min is comparable in retention times and RRT to desosaminylazithromycin, with a specified RRT of 0.54 in the monograph (Table 2). Thus, providing peak confirmation of desosaminylazithromycin on the Alliance iS HPLC. In contrast, when analyzing the data from System Z there is no clear peak in either retention time or RRT indicative of desosaminylazithromycin. Due to the retention time variability, confirmation based on retention time or the RRT of desosaminylazithromycin is challenging and may lead the analyst to the wrong conclusion.

Conclusion
The USP provides monographs for the purpose of providing pharmaceutical quality assurance. In the case of HPLC monographs, system parameters and system suitability requirements are detailed in order to generate complaint and reliable data. While many HPLC systems from various vendors can meet the minimum system requirements needed to execute an assay, it is not uncommon to see differences in data sets generated using varying instruments. While all systems tested showed acceptable system suitability results, system performance varied from system to system. In this study, the Alliance iS HPLC System was shown to be the most robust and reproducible system in comparison to the others evaluated. On the Alliance iS HPLC System, the system suitability requirements were easily met and showed the lowest retention time variability from injection to injection, resulting in impurity identification confidence.
References
- United States Pharmacopeia (2022) USP Monographs Azithromycin Organic Impurities, USP-NF. Rockville, MD:USP DOI:https://doi.org/10.31003/USPNF_M6740_05_01
720008116, November 2023