
Metal adsorption can be a significant problem in LC analysis, particularly for highly acidic probes like phosphorylated peptides, or small molecules such as nucleotides. These probes can interact with chromatographic metal surfaces, leading to poor peak shape, reduced peak area, or even complete loss of recovery.1 This issue can be mitigated by implementing MaxPeak High Performance Surfaces (HPS) Technology, which has been applied to Waters LC columns and LC systems. However, competitive LC systems do not employ this technology. Here, we demonstrate the use of MaxPeak Premier Columns on three different, non-Waters LC systems for the analysis of a metal-sensitive analyte, adenosine 5’-(α,β-methylene) diphosphate (AMPcP). This probe has shown strong metal-sensitivity and is more stable than compounds such as adenosine triphosphate or adenosine diphosphate.2 Our results show that regardless of the LC system used, a MaxPeak Premier Column yielded higher peak areas and thus analyte recovery for AMPcP compared to stainless steel columns. This indicates that the advantages of the MaxPeak Premier Columns are system-agnostic and would prove beneficial to almost all system configurations.
Metal surfaces in an LC system, including column hardware, can interact with acidic analytes through Lewis acid-base attraction, which can lead to poor peak shape and recovery. The interaction can also take place for basic or neutral compounds, through a different mechanism, but are more common for acidic probes. This effect can be minimized by using high pH mobile phases, as metal surfaces are positively charged in neutral and acidic pH environments. However, even at high pH this effect can still be problematic. To combat this, MaxPeak High Performance Surfaces (HPS) have been applied to many of the column chemistries offered by Waters as well as some LC systems, including the ACQUITY Premier System. By using a MaxPeak Premier Column on the ACQUITY Premier System, metal interactions can be minimized, allowing for high recovery and good peak shape of acidic probes.1,3–5 While MaxPeak Premier Columns provide the largest benefit when coupled with an ACQUITY Premier System, the columns can also be used on any LC system to improve the recovery of acidic probes.
Thus, the work performed for this application demonstrates that XSelect Premier HSS T3 Columns can provide increased recovery for an acidic probe on non-Waters LC systems versus conventional stainless steel column technology. An Agilent 1290 Infinity I, a Shimadzu Nexera-I LC2040, and a Thermo Vanquish UHPLC System were evaluated using a standard containing equimolar concentrations of AMPcP and adenosine to investigate the metal interactions of the three LC systems. AMPcP, a compound with two phosphate groups, is sensitive to metal surfaces but is non-hydrolyzable and thus more stable than a nucleotide probe. Adenosine is a structurally similar compound but without the phosphate groups allowing similar UV response, but without potential metal interactions. From this study, the XSelect Premier HSS T3 Column produced the highest recoveries regardless of the system used, where in one case, recoveries were >50% higher than the conventional columns tested.
Waters AMPcP and Adenosine Standard (P/N: 186009755) was reconstituted in 200 µL Milli-Q Water. The vial was then vortexed for 10 seconds prior to injection. A fresh sample from the same lot was created prior to analysis on each system.
|
LC Conditions |
|
|
LC systems: |
Agilent 1290 Infinity I Thermo Vanquish UHPLC Shimadzu Nexera-I 2040 |
|
Detection: |
UV @ 260 nm |
|
Vials: |
N/A |
|
Column(s): |
XSelect HSS T3 XP Column, 2.1 x 50 mm, 2.5 µm (PN: 186006149) XSelect Premier HSS T3 Column, 2.1 x 50 mm 2.5 µm (PN: 186009830) Zorbax SB-Aq Column, 2.1 x 50 mm, 3.5 µm |
|
Column temp.: |
40 °C |
|
Sample temp.: |
Ambient |
|
Injection volume: |
1 µL (3 µL on Shimadzu Nexera-I) |
|
Flow rate: |
0.36 mL/min (0.70 mL/min on Shimadzu Nexera-I) |
|
Mobile phase A: |
10 mM Ammonium acetate pH 6.8 in 99.8/0.2 water/acetonitrile |
|
Mobile phase B: |
8 mM Ammonium acetate pH 6.8 in 79.8/20.2 water/acetonitrile |
|
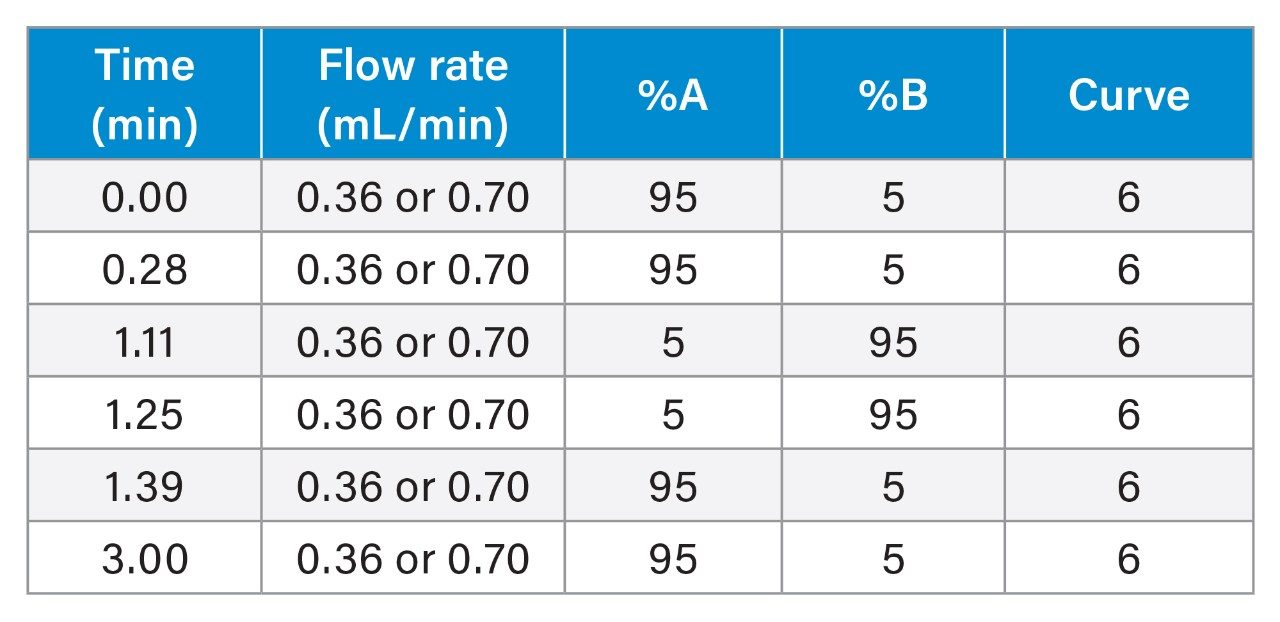
Gradient: |
See table |
|
Chromatography software: |
Empower 3 Feature Release 5 (Agilent and Shimadzu Systems) or Chromeleon Software version 7.2 (Thermo System) |
AMPcP has been previously employed as a quality control standard to characterize the extent of metal interactions on systems with and without MaxPeak HPS.2 In combination with a structurally similar, yet non-reactive compound like adenosine, the peak areas of both the metal-sensitive AMPcP and insensitive probes can be compared. The standard used here employs equimolar concentrations of adenosine and AMPcP allowing for direct comparisons between peak areas and calculating a peak area ratio, which can be used to assess the metal interactions in a system. Therefore, we applied this standard to verify that the performance advantages of MaxPeak Premier Columns are system-agnostic by evaluating alternative LC systems without HPS. In order to do so, the documented LC conditions for the standard had to be transferred to the test column configuration. The method was changed by using the ACQUITY Columns Calculator and applying the correct analysis conditions to the new columns. Prior to testing, each column was equilibrated to starting conditions for 10 minutes, and a blank injection was performed. The first system tested was an Agilent 1290 Infinity I, equipped with a quaternary pump, and DAD detection. Figure 1 shows the first chromatogram of the AMPcP adenosine standard on the Agilent 1290 system.
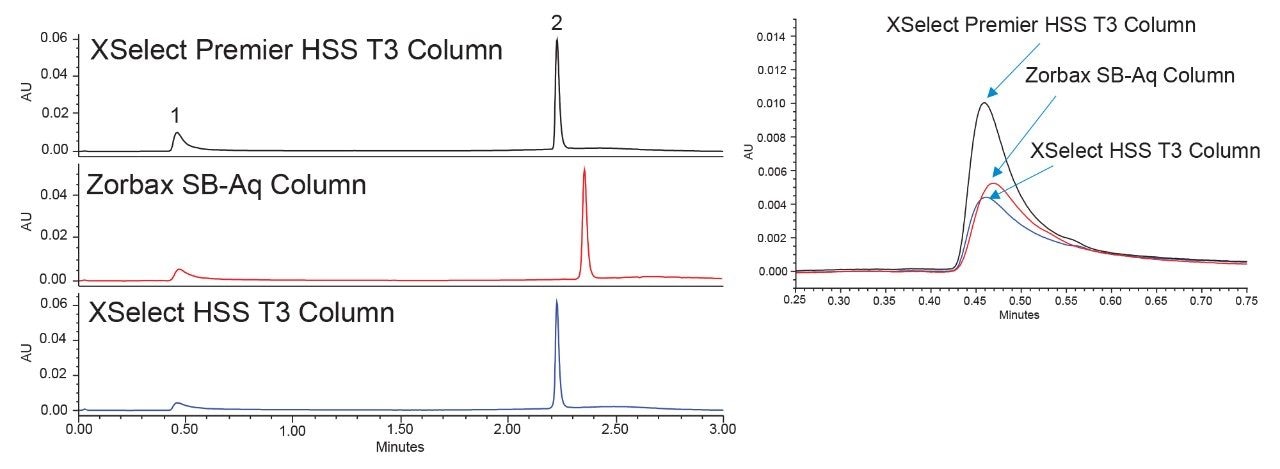
The AMPcP peak is considerably higher on the XSelect Premier HSS T3 Column compared to the other two columns. This can be directly attributed to the MaxPeak HPS hardware of the column as the XSelect HSS T3 contains the same stationary phase but different column hardware. The Zorbax SB-Aq column shows slightly higher peak height compared to the XSelect HSS T3, but not as high as the XSelect Premier HSS T3 Column. Additionally, as shown in Table 1, the peak areas for AMPcP are greatest on the XSelect Premier HSS T3 Column.
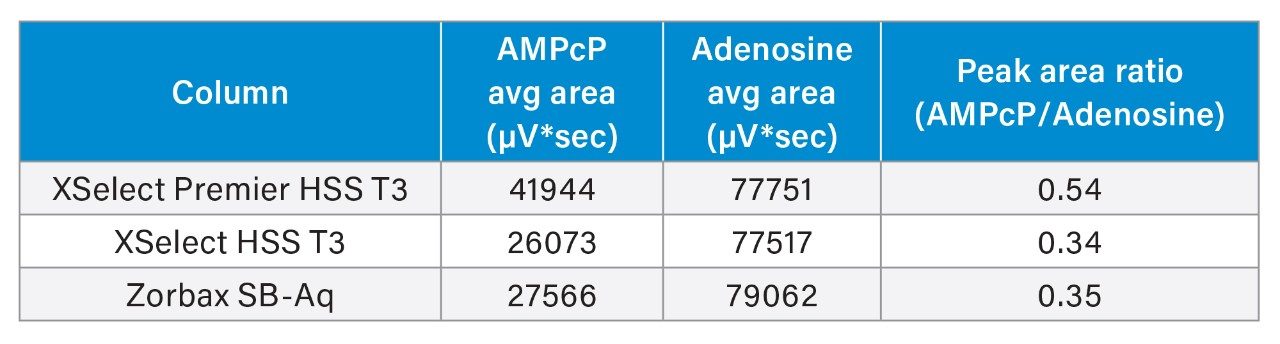
The XSelect Premier HSS T3 Column yielded an average peak area for AMPcP >50% higher than the other columns tested. Meanwhile, the peak areas for adenosine are consistent across the three tested columns. The decreased peak area for AMPcP on the stainless steel columns can be directly attributed to the loss of analyte due to metal interaction. The two columns in stainless steel hardware performed similarly, with peak area ratios of 0.34 and 0.35, while the XSelect Premier HSS T3 Column gave a peak area ratio of 0.54. The higher peak area ratio indicates different levels of metal adsorption for each column. The higher the peak area ratio, the less adsorption is detected.
A Shimadzu Nexera-I LC2040 configured with a quaternary pump and UV detection was investigated next. During initial testing, the retention of adenosine was higher than anticipated and a change to the method was needed. This can be attributed to both gradient delay differences for the system, as well as system configuration. The flow rate was increased to 0.7 mL/min on the Shimadzu system. Additionally, due to low UV signal for adenosine and AMPcP on this system, the injection volume was increased to 3 µL. Getting good UV signal for both probes is essential to determining the peak area ratios and assessing the metal interactions for the columns. Figure 2 shows the chromatography achieved on the Shimadzu Nexera-I using the three columns.
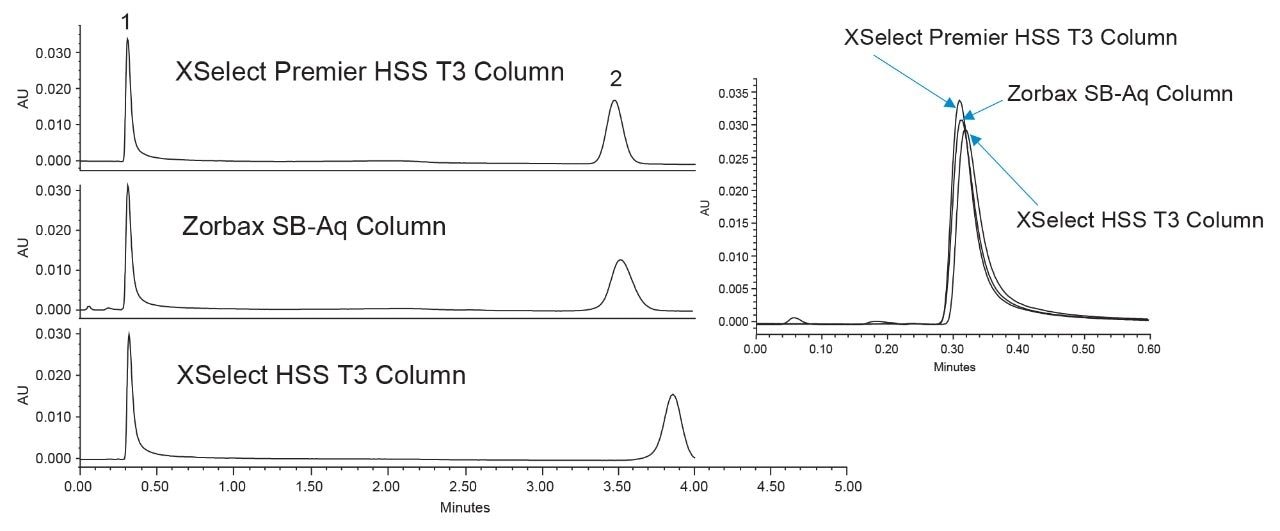
At first glance, the three columns, perform comparably with similar peak heights, though the XSelect Premier Column is slightly higher, as was seen on the Agilent system. This could be due to the increased injection volume of the standard on the Shimadzu system, which was needed to achieve reasonable peak heights. It has been shown that at different mass loads, i.e. mass of analyte injected onto the column, the adsorptive affect is different.1,3,7 While the difference between the three columns is subtle based on the chromatography, there is still an increase in peak area when using the XSelect Premier HSS T3 Column. Table 2 shows the average peak areas for the replicate injections of standard on the Shimadzu Nexera-I.
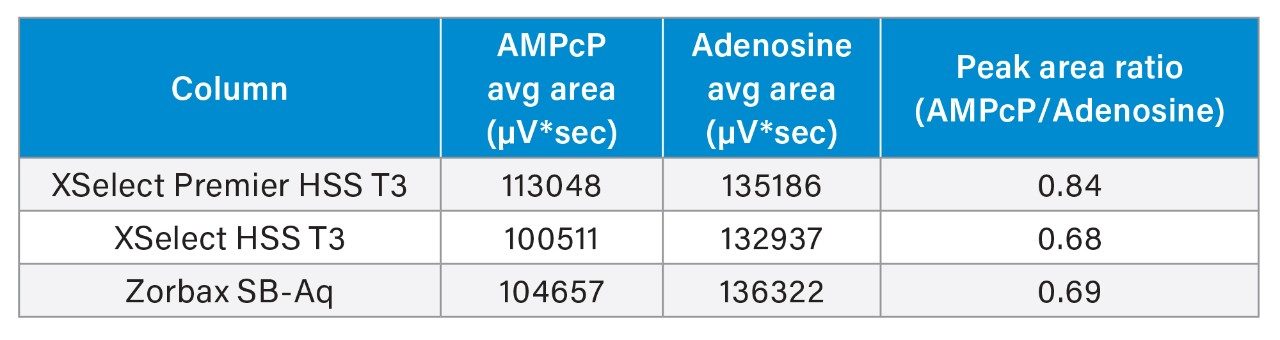
The XSelect Premier HSS T3 Column yielded an average peak area 10% higher than the other two columns, and a peak area ratio almost 0.2 units higher. This would indicate that while the chromatography looks comparable between the three columns, the XSelect Premier HSS T3 Column still provides a benefit in analyte recovery.
The last system to be tested was a Thermo Vanquish equipped with a quaternary pump and UV detection. Chromeleon 7.2 was used for software control. Figure 3 shows the chromatography obtained on the Thermo Vanquish system using the three columns and the AMPcP adenosine standard.
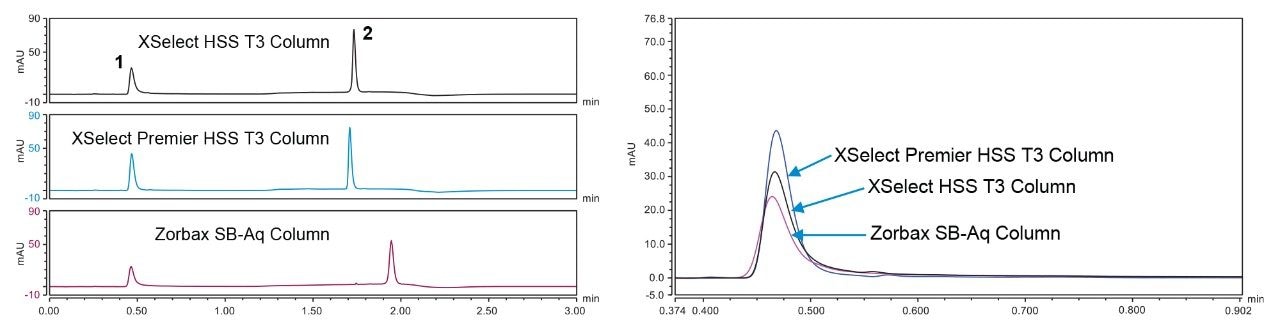
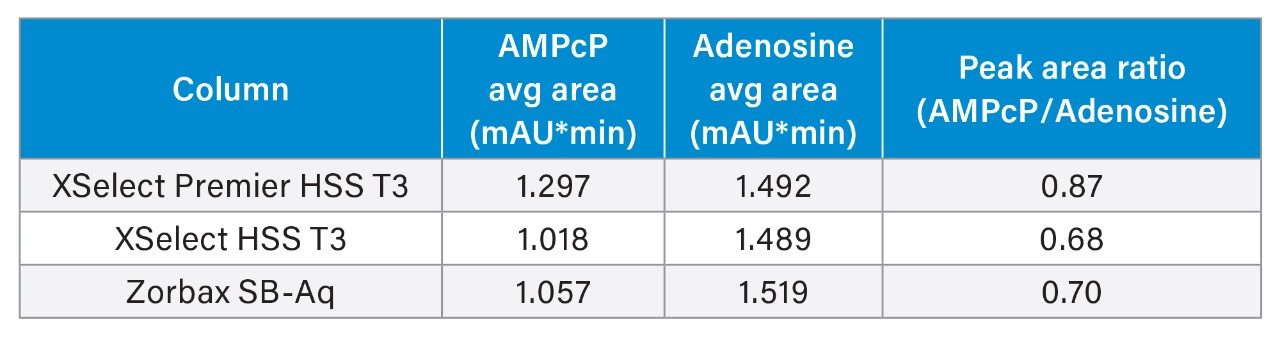
On the Thermo Vanquish system, the XSelect Premier HSS T3 Column produced 20% higher peak areas than the other two columns. The peak area ratio is also higher on the XSelect Premier HSS T3 Column by almost 0.2 units.
Three LC systems were used to test three different columns for the extent of metal interaction present. In order to test the metal interactions a metal sensitive probe, AMPcP, was analyzed with an insensitive probe, adenosine. When analyzed together, the peak areas of the two probes can be compared obtaining a peak area ratio. This ratio is a measure of how the metal interactions possibly at play within the system. Higher peak area ratios indicate less metal interaction.
The peak area ratios obtained in this work show that MaxPeak Premier Columns can provide increased peak recovery for an acidic probe compared to stainless steel columns. The use of MaxPeak Premier Columns can help analysts detect and quantify acidic analytes regardless of what LC system is being used, without the need for passivation agents or specialized separation methods. This allows for more accurate quantitation and potentially lower limits of detection, both of which are critical for certain assays.
720007316, July 2021