This application note demonstrates how Auto•Blend Plus Technology facilitates IEX method development and robustness testing.
Auto•Blend Plus Technology facilitated IEX method development and robustness testing.
Ion-exchange chromatography (IEX) is widely used for the charge-variant analysis of protein biotherapeutics. Its ability to distinguish between isoforms with different surface charge, including species that have the same isoelectric point (pI), and to collect fractions for further analysis has great utility in the biopharmaceutical industry.1,2 Optimization of an IEX method for each protein therapeutic drug involves parameter considerations.3 An intelligent method setup can significantly reduce the time spent for method development and robustness testing.
Waters Auto•Blend Plus Technology uses concentrated solvents to blend mobile-phase compositions at a specific pH. At the same time, it controls the concentration of salt to optimize separations. It is available in Waters’ four-solvent deliver systems (i.e., ACQUITY UPLC H-Class, ACQUITY UPLC H-Class Bio, ACQUITY Arc, and ACQUITY Arc Bio) to deliver mobile-phase compositions. As illustrated in Figure 1, only four bottles of solvent are needed to deliver the desired gradient by pH and salt concentration: concentrated acid, base, salt, and water.
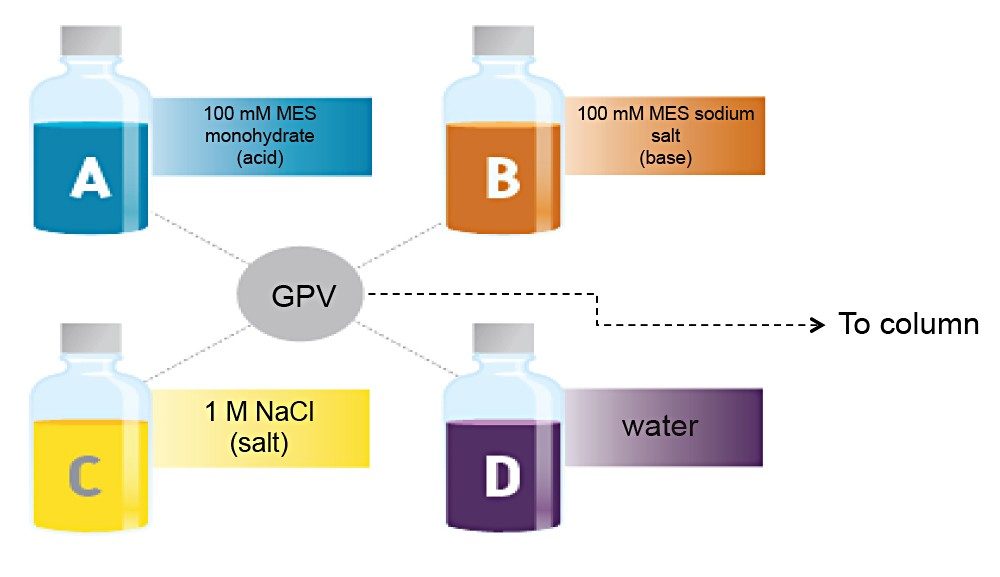
In this application note, we show how Auto•Blend Plus Technology facilitates IEX method development and robustness testing.
Trastuzumab (analyzed past expiry) was diluted in water to 5 mg/mL, mAb Charge Variant Standard (p/n: 186009065) was diluted in 100 μL of water.
|
System: |
ACQUITY UPLC H-Class Bio |
|
Sample temp.: |
10 °C |
|
Analytical column temp.: |
30 °C |
|
Flow rate: |
0.8 mL/min for trastuzumab; 1.2 mL/min for mAb Charge Variant Standard |
|
Injection volume: |
1 μL for trastuzumab; 25 μL for mAb Charge Variant Standard |
|
Column: |
BioResolve SCX mAb, 3 μm, 4.6 × 50 mm (p/n: 186009058) |
|
Detection: |
ACQUITY UPLC TUV with 5 mm titanium flow cell, 280 nm |
|
Sample collection/vials: |
LCGC Certified Clear Glass 12 × 32 mm Screw Neck Total Recovery Vial, with Cap and Preslit PTFE/Silicone Septa, 1 mL volume (p/n: 186000385C) |
|
Mobile phase A: |
100 mM MES monohydrate |
|
Mobile phase B: |
100 mM MES sodium salt |
|
Mobile phase C: |
1 M NaCl |
|
Mobile phase D: |
Water |
|
Data management: |
Empower 3 |
Auto•Blend Plus functionality is embedded in Empower Software. Figure 2 compares the conventional instrument method with the Auto•Blend Plus method. In the conventional method, percent A, B, C, and D is used to build a gradient method. In the Auto•Blend Plus method, a buffer system is pre-defined, and pH and salt concentration values can be directly put into the gradient method (the blue boxes). To convert a conventional method to an Auto•Blend Plus method, select the “Auto•Blend Plus” button (the red box). Please note that the Auto•Blend Plus method cannot be converted back to the conventional method, so it is a good idea to save the method under a different name.
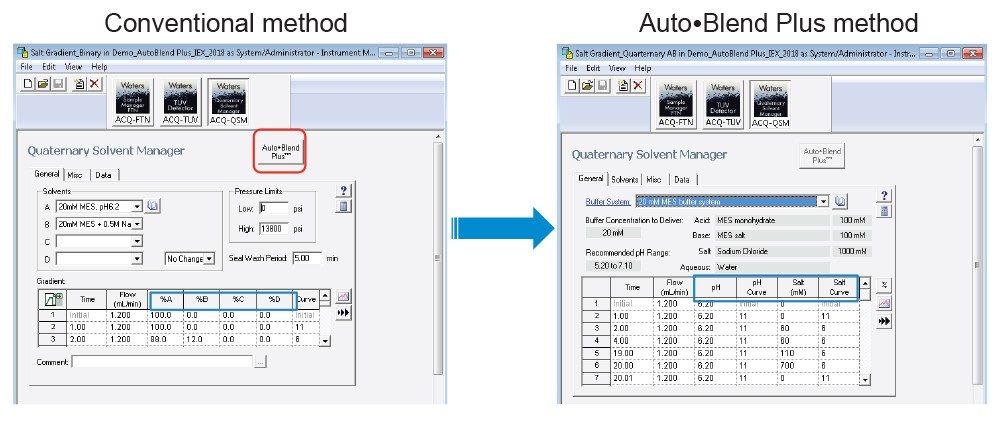
One of the advantages of using Auto•Blend Plus Technology is that it can speed up method development by saving mobile-phase preparation time significantly and making the method programming easier and less prone to error.
Figures 3A and 3B show an example of pH optimization of trastuzumab, a monoclonal antibody, in a salt gradient separation. Because the buffer system is pre-defined, when the pH and salt concentration values are plugged into the table, the software algorithm will calculate how to deliver the required pH and salt concentration. For example, if the pKa of a buffer system is given, then the software will calculate the percentage of acid and base needed to deliver a certain pH based on the Henderson-Hasselbalch equation. Alternatively, taking into consideration the impact of salt on pH, an empirical table can be constructed by mixing different proportions of acid and base with different salt concentrations and measuring the pH values of each solution. The software will then calculate the composition of percent acid and base, based on the pH values in the empirical table. To screen pH using the Auto•Blend Plus method, only the pH value needs to be changed in each method, and only the four concentrated solvents will be used. If a conventional method were to be used for the pH screening, up to twelve buffers would need to be made and then titrated to the desired pH.
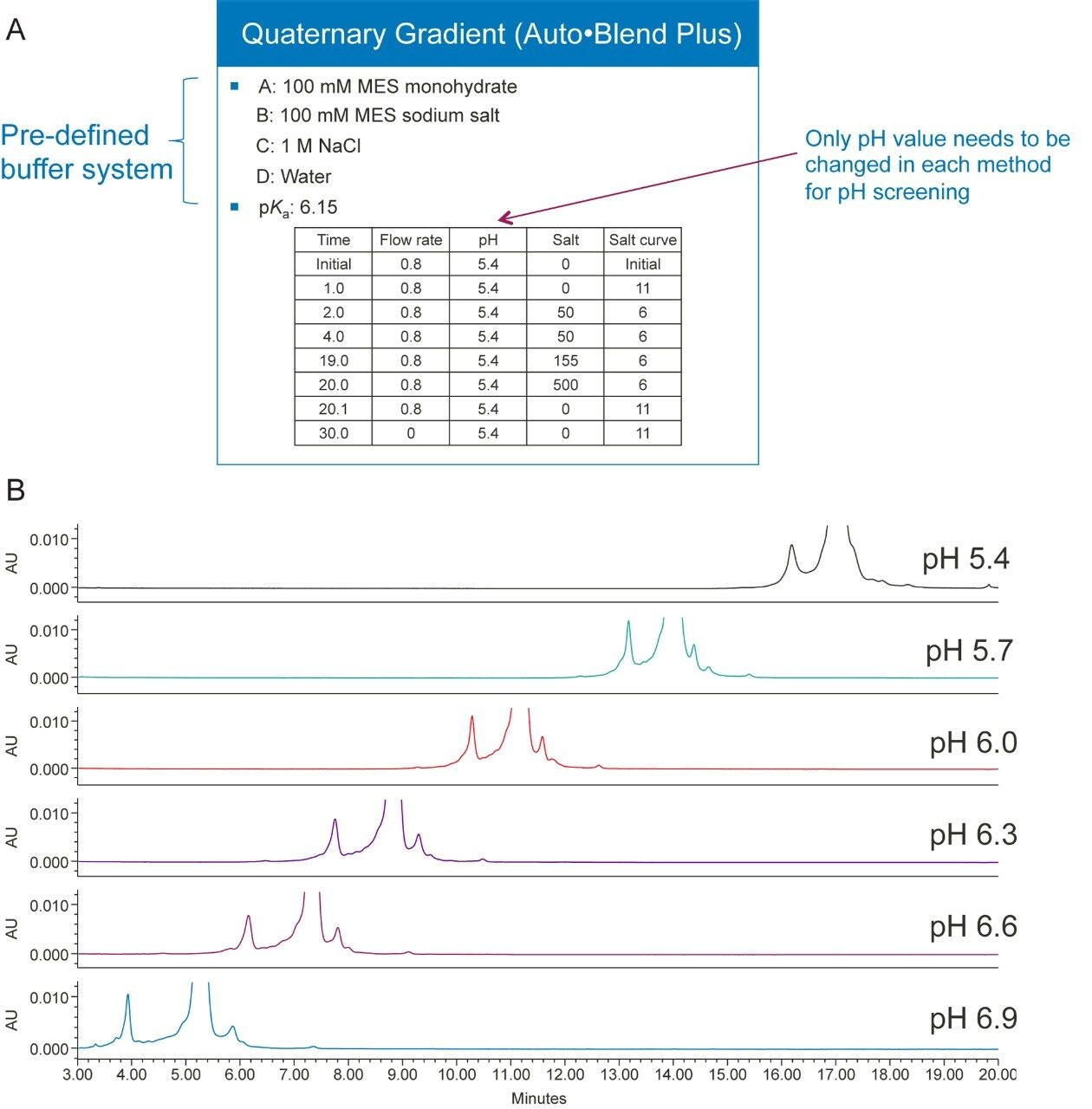
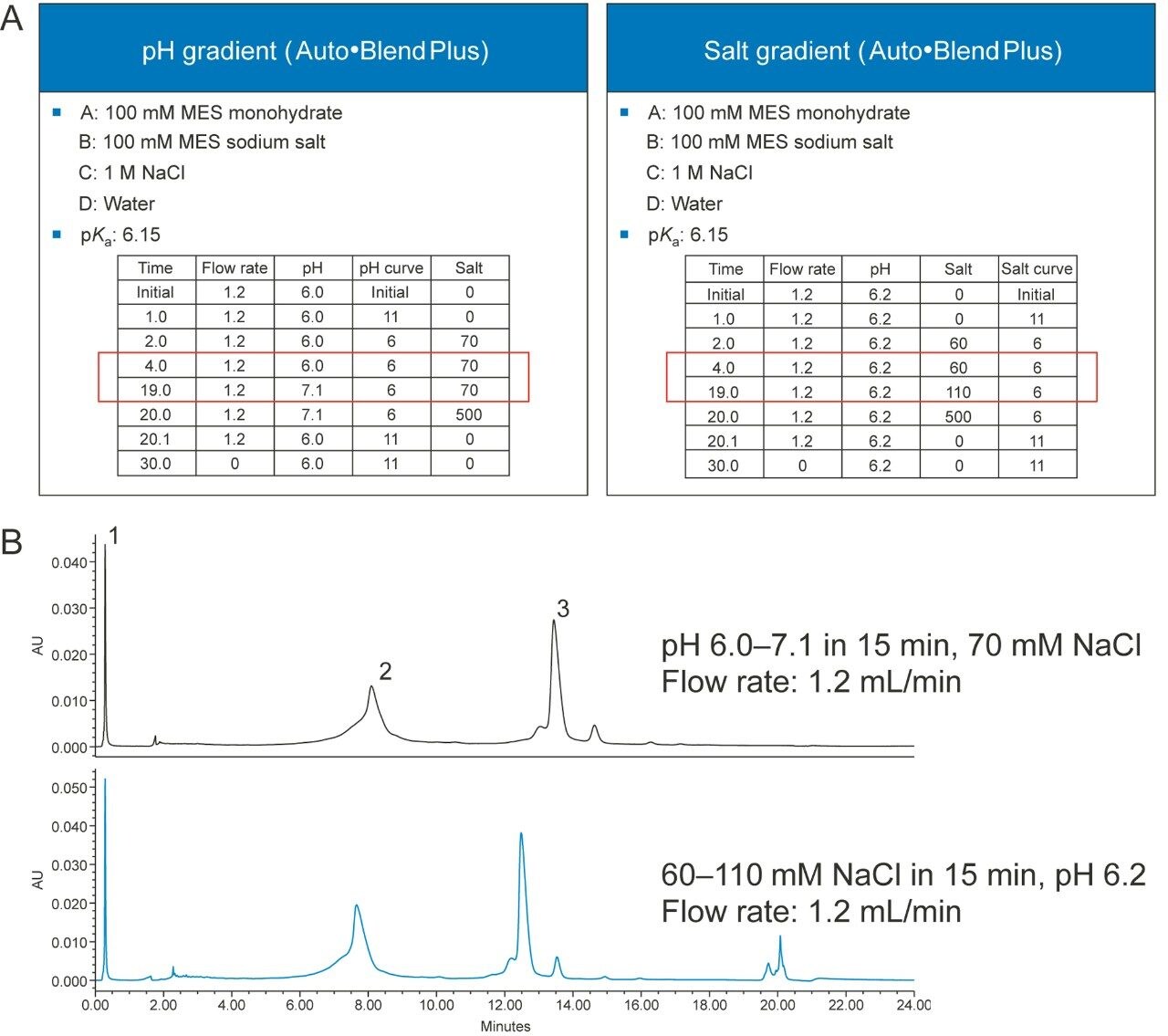
Figure 4A shows two gradient tables, one is a fixed-salt pH gradient table, and the other is a fixed-pH salt gradient table. Figure 4B shows both methods provide adequate separation for the mAb Charge Variant Standard which contains three major components: tryptophan, conalbumin, and NIST mAb. Not shown in the examples are dual pH/salt gradients that can be easily setup in the gradient table of the Auto•Blend Plus methods. Please note that all of these methods can be run by manipulating the Auto•Blend Plus gradient table without changing any solvents in the mobile phases.
A very important advantage of Auto•Blend Plus Technology is that it helps define the robustness range of a method.
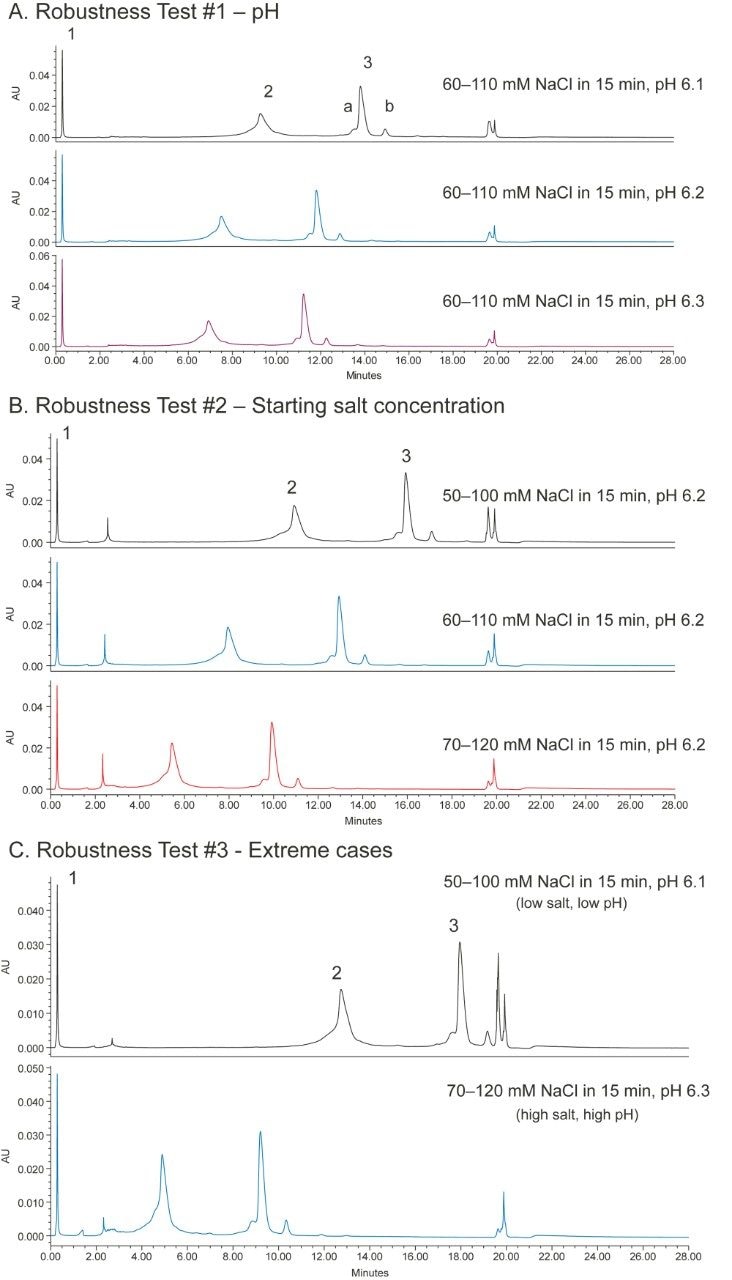
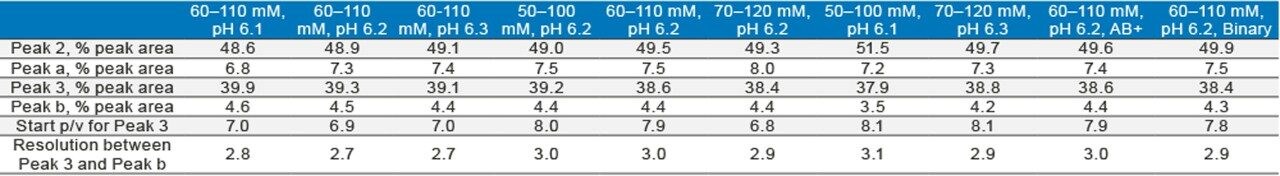
The robustness of a method can be tested by separating the analyte under conditions where the pH and salt concentration are changed to some extent. Figure 5A shows the effect of small changes in mobile-phase pH on the separation of the mAb Charge Variant Standard; Figure 5B shows the effect of changing the salt concentration of the gradient; and, Figure 5C shows two extreme cases. As can be seen, a small change of the mobile-phase conditions did not impact the separation significantly. Table 1 shows the percent peak area of four major peaks as well as resolution or peak-to-valley ratio between two adjacent peaks under various conditions in the robustness test. The values are comparable in most cases. Based on the results, it is concluded that the method gives good separation in the 55–115 mM salt concentration range under pH 6.1–6.3. Since all of the methods can be made by simply changing the pH and salt concentration values, and no more solvents need to be made, the robustness range can be found in a short period of time.
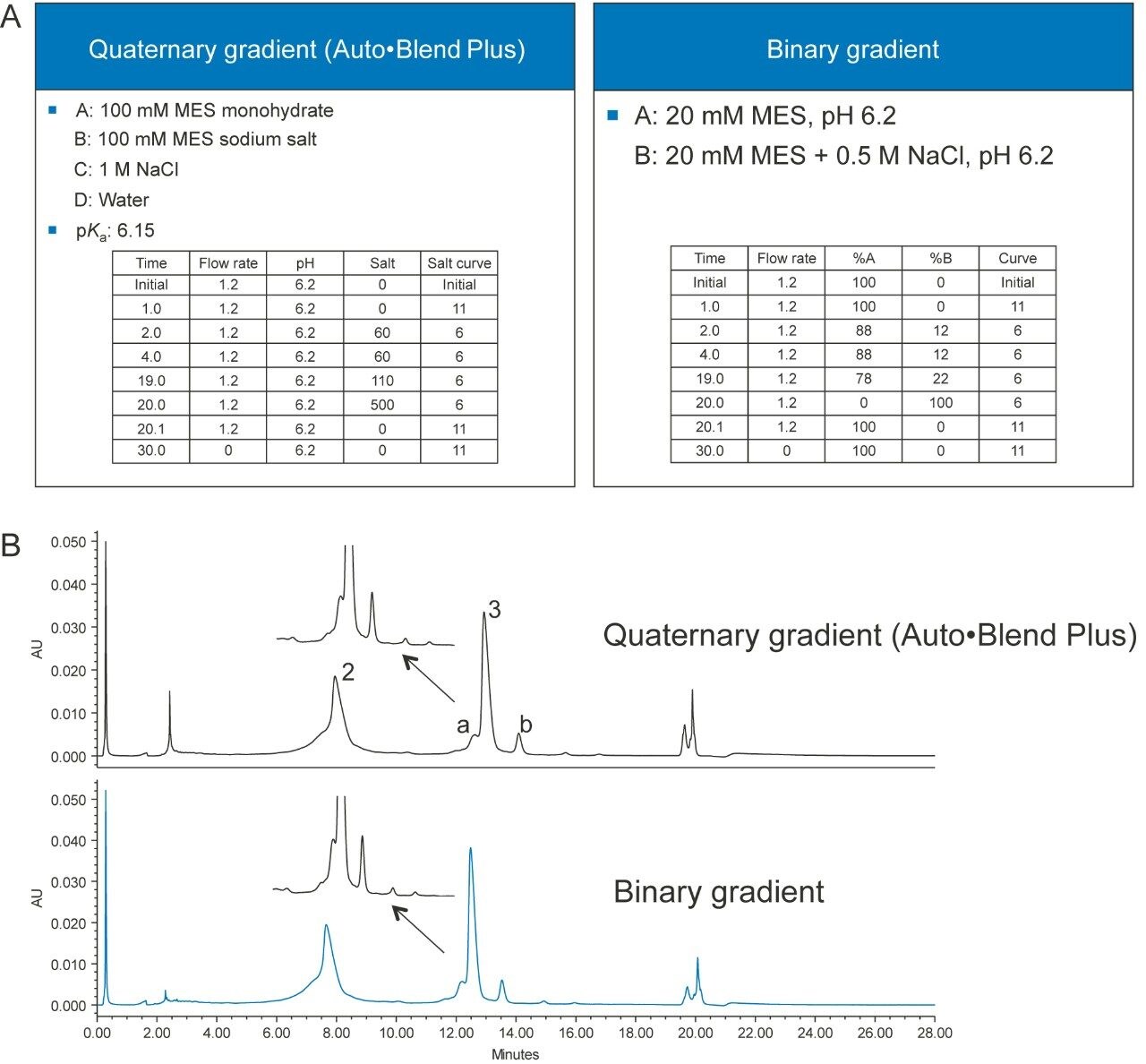
The Auto•Blend Plus method can be transferred to a conventional method, if the instrument where the method will be transferred to does not support Auto•Blend Plus Technology. Figure 6A shows a quaternary Auto•Blend Plus method and a conventional binary method, and Figure 6B shows the chromatograms obtained from using both methods.
The separation is very similar using both the Auto•Blend Plus and conventional compositional methods (Table 1).
Waters Auto•Blend Plus Technology automates the formulation of mobile phases from concentrated solvents using quaternary pumping systems. It not only simplifies the method development process, but also helps to quickly define the method robustness range. It is an excellent tool for ion-exchange chromatography method development and robustness testing, where the charge-variant separation is sensitive to pH and salt concentration.
For more information on how to use Auto•Blend Plus Method in Empower Software, please visit marketplace.waters.com and download the “Auto•Blend Plus Guide for IEX”.
720006557, April 2019