
This study demonstrates an integrated workflow for targeted and non-targeted screening using UPLC and GC on a single MS platform with UNIFI informatics for extractable and leachable screening in e-cigarettes, food, cosmetics, and pharmaceutical packaging applications.
Characterization of extractables and leachables is essential for ensuring the safety, quality, and efficacy of inhalation tobacco products such as e-cigarettes. The initial step for characterizing extractables from e-cigarettes involves targeted screening where you analyze the extract and quantify against known impurity standards. This is a well-established process that can be performed using analytical techniques such as GC-MS, LC-MS/MS and ICP-MS. However the finished products (e-liquids, refill cartridges, and e-cigarette aerosol) may have impurities present from the starting materials and other packaging and device components that need to be further evaluated by non-targeted screening analysis.
E-cigarette regulations are still evolving due to a lack of scientific information and lack of product quality and safety standards. Both the US FDA regulation and the revised EU Tobacco Products Directive (TPD2; 2014/40/EU) subject e-cigarette manufacturers to product and ingredient disclosures and good manufacturing practices to ensure e-cigarette products are appropriate for the protection of the public health.1,2 In the UK, the MHRA (Medicines and Healthcare Products Regulatory Agency) regulates e-cigarettes as nicotine delivery devices and requires manufacturers to provide complete quality information for licensing e-cigarette devices including the composition of the e-cigarette device, the plastic, polymer, and metal components used, the quality of the nicotine and excipients, data from extractables and leachables studies, and product stability data during use, and shelf-life.3
In this study, the various components of an e-cigarette device (end caps, mouth piece, gauze, heating element, and flavor formulation) were extracted individually and subjected to non-targeted high resolution screening using UPLC and GC which can be configured to the same QTof-MS. Accurate mass data for precursor and fragment ions was acquired using alternating high and low collision energy states (MSE) across the full analytical mass range. Data from the sample component extracts was compared to the reagent blank to determine differences and identify potential extractables. In this application note, we describe a workflow on how non-targeted screening for extractables and leachables testing can be performed in e-cigarettes. The workflow demonstrated here is also applicable to nontargeted screening for extractables and leachables in packaging for food, cosmetics, and pharmaceuticals.
The various components of a closed system e-cigarette cartridge (outer and inner end caps, mouth piece, gauze with flavor formulation, paper wrap, and metal shell) were extracted separately using isopropanol solvent for 30 minutes and subjected to non-targeted high resolution screening using UPLC and GC coupled to QTof-MS. As part of the batch QC analysis, Waters Extractables and Leachables Screening Standard [p/n: 186008063], that includes 18 common polymer additives, was used to evaluate and benchmark the high resolution UPLC-QTof-MS system. The Extractables and Leachables Screening Standard covers a mass range of up to 1176 Da, supporting both positive and negative ionization modes.
|
UPLC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC BEH C18, 130Å, 1.7 μm, 2.1 × 100 mm |
|
Column temp.: |
45 °C |
|
Sample temp.: |
4 °C |
|
Mobile phase A: |
10 mM ammonium acetate (pH 5.0) in water |
|
Mobile phase B: |
10 mM ammonium acetate (pH 5.0) in water |
|
Flow rate: |
0.45 ml/min |
|
Needle wash: |
50:50 water:methanol (v/v) |
|
Syringe purge: |
10:90 methanol:water (v/v) |
|
Total run time: |
17 min |
|
Injection volume: |
10 μL |
|
Time (min) |
%A |
%B |
|---|---|---|
|
0.00 |
98 |
2 |
|
0.025 |
98 |
2 |
|
12.25 |
1 |
99 |
|
13.00 |
1 |
99 |
|
13.01 |
98 |
2 |
|
17.00 |
98 |
2 |
|
MS system |
Xevo G2-XS QTof |
|
Capillary voltage |
0.8 kV |
|
Sampling cone |
20.0 |
|
Source temp. |
120 °C |
|
Source offset |
80 |
|
Carrier gas |
Nitrogen |
|
Cone gas flow |
50 L/Hr |
|
Desolvation gas flow |
1000 L/Hr |
|
Acquisition range |
50–1200 m/z |
|
Scan time |
0.25 sec |
|
Lockmass |
Leucine enkephalin (556.2771 m/z) |
|
GC system: |
A7890 (with APGC Interface) |
|
Column: |
DB-5MS 0.25 μm, 30 m × 0.25 mm |
|
Desolvation temp.: |
550 °C |
|
Flow rate: |
1.2 mL/min |
|
Initial temp.: |
35 °C (1.6 min) |
|
Ramp: |
25 °C/min |
|
Final temp.: |
320 °C (7 min) |
|
Run time: |
20 min |
|
Inlet mode: |
Splitless |
|
Inlet type: |
Multimode |
|
Temp.: |
280 °C |
|
Injection volume: |
1 μL |
|
Make-up gas: |
Nitrogen |
|
Make-up gas flow: |
250 mL/min |
|
Transfer line temp.: |
310 °C |
|
QTof System: |
Xevo G2-XS QTof MS (with APGC interface) |
|
Corona current: |
3.0 μA |
|
Sampling cone: |
20.0 |
|
Source temp.: |
120 °C |
|
Source offset: |
80 |
|
Cone gas flow: |
175 L/Hr |
|
Auxiliary gas flow: |
50 L/Hr |
|
Acquisition range: |
50–1200 m/z |
|
Scan time: |
0.25 sec |
|
Lockmass |
Siloxane bleed (281.0517 m/z) |
Accurate mass data from both the GC and UPLC–QTof-MS analysis of the e-cigarette component extracts were acquired and processed using the UNIFI Scientific Infomation System.
The Xevo G2-XS QTof-MS couples to either UPLC or GC to provide a full system solution for chemical profiling. Accurate mass data from both the GC and UPLC-QTof-MS analysis of e-cigarette component extracts were acquired and processed using the extractables and leachables workflow in the UNIFI Scientific Information System. Precursor and fragment ions were acquired simultaneously using alternating low- and high-collision energy states (MSE) across the full analytical mass range. Potential candidate markers were screened against a library of known extractables and leachables compounds in UNIFI, and automatically interrogated using multiple matching criteria including accurate mass for precursor and fragment ions, adducts, and isotopic fit.
The GC-QTof-MS profiles of e-cigarette component extracts are shown in Figure 1. Potential extractables were short-listed based on the following criteria: detector response >1000, mass error ± 5 ppm and the number of expected fragments detected >0. The established UNIFI workflow utilizes accurate mass precursor and fragment ion data, and applied criteria to simplify data review and facilitate the decision-making process. It allows analysts to evaluate complex data in a more efficient way and enables rapid identification of known and unknown compounds.
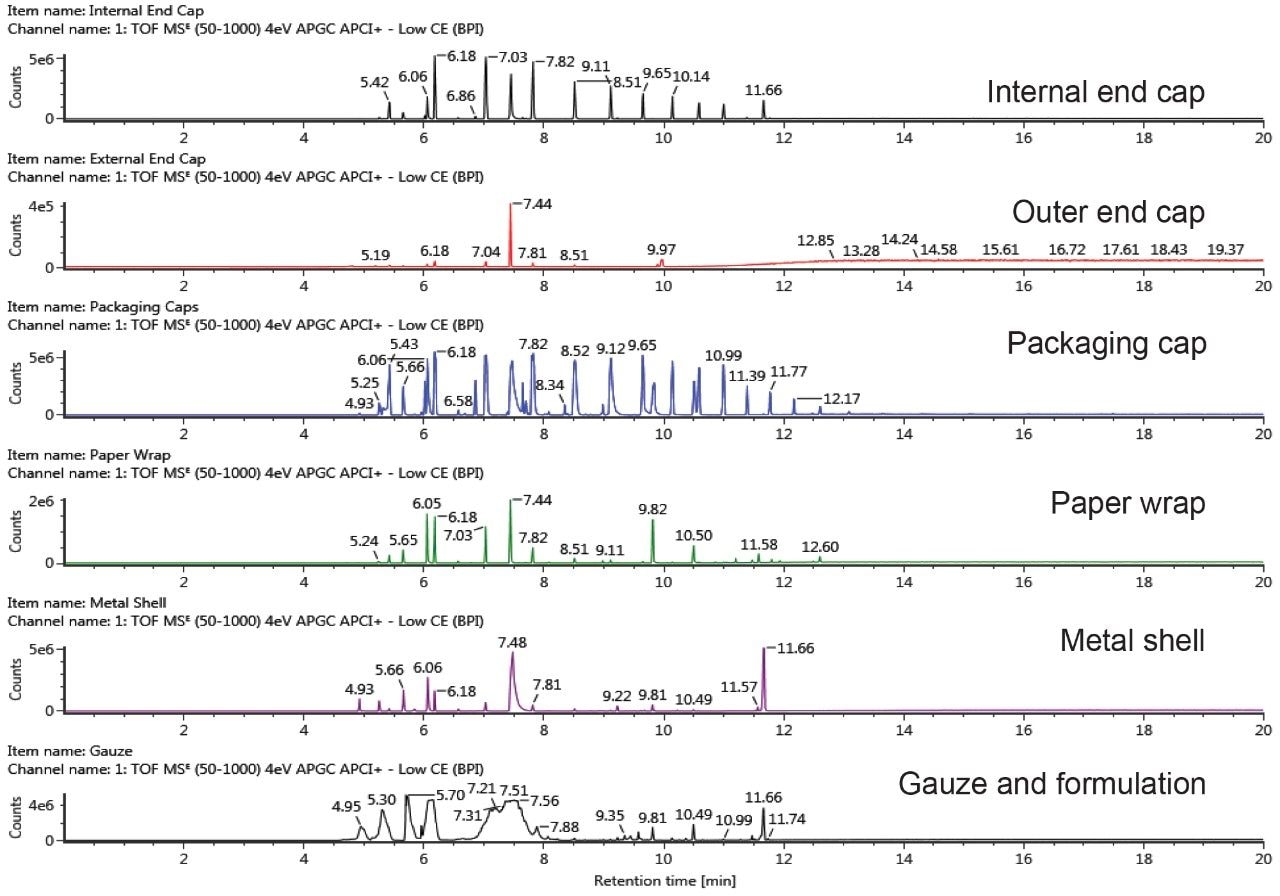
Figure 2 exhibits the identification of dibutyl phthalate (DBP), a common plasticizer, in the internal end cap, metal shell, and gauze extracts using GC-QTof-MS analysis. The DBP peak had a high detector response (>11,000) in the component extracts compared to the solvent blank, one identified fragment ion, and a low measured mass error (<2.5 ppm). The migration of DBP across the internal end cap, metal shell, and gauze is possible as these components come in contact with each other in the e-cigarette cartomizer assembly.
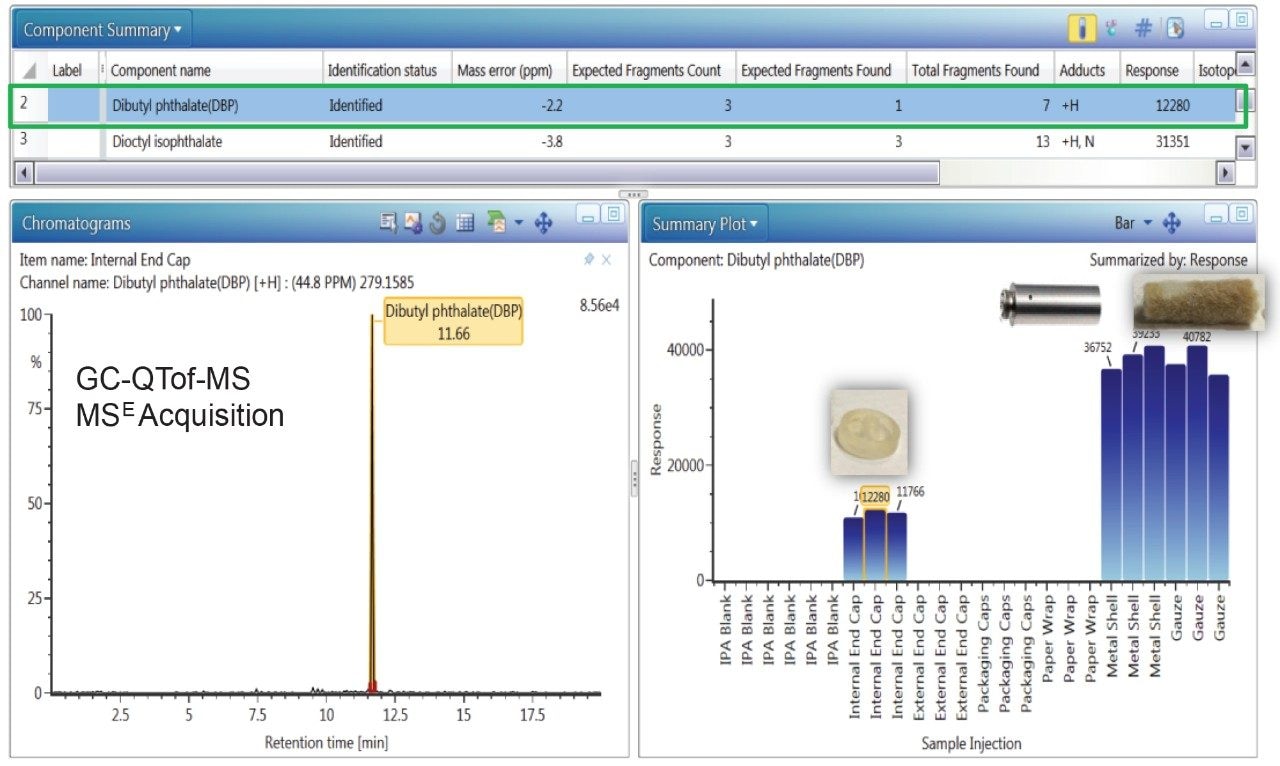
Figure 3 shows the identification of HMBTAD, a light stabilizer in the internal end cap, metal shell, and gauze extracts using UPLC-QTof-MS analysis. The HMBTAD peak had a high detector response (>42,000), low mass error (<1.5 ppm) and was not identified in solvent blanks. The relative levels of HMBTAD are higher in the gauze containing the flavor formulation, potentially to increase the product shelf-life stability.
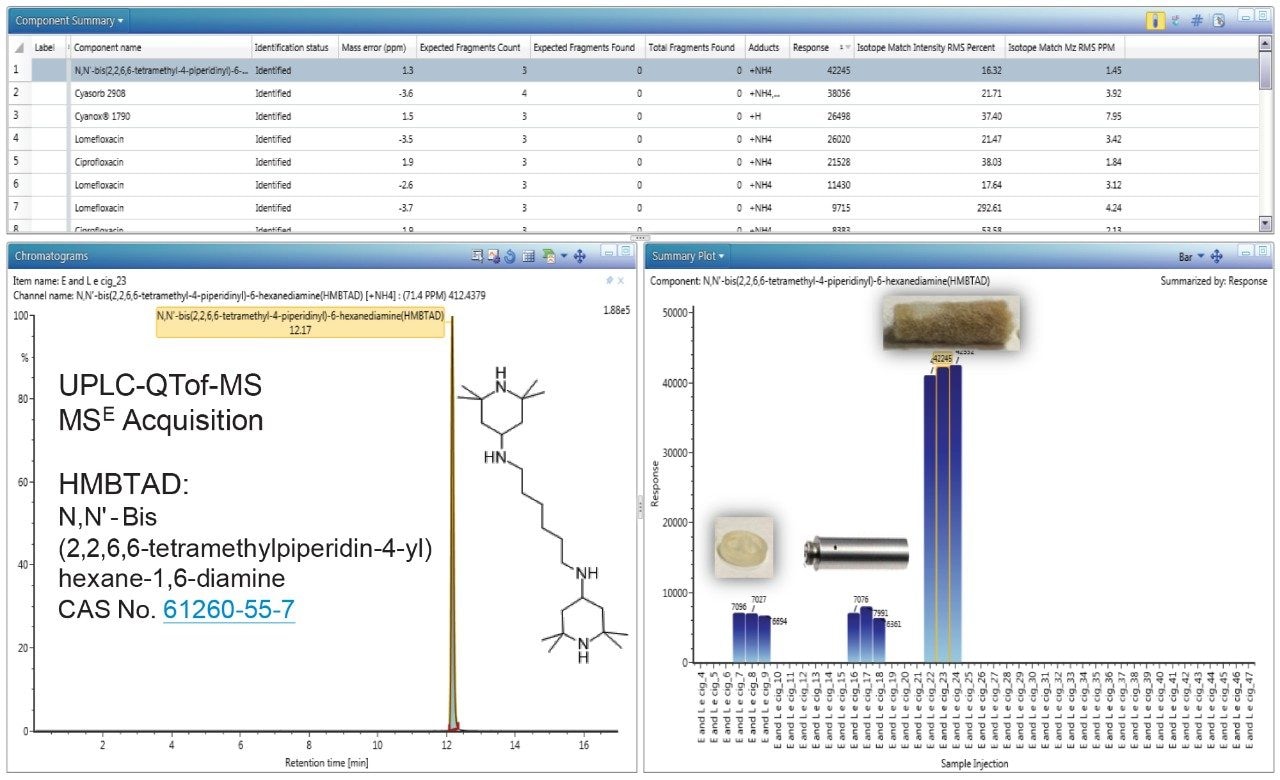
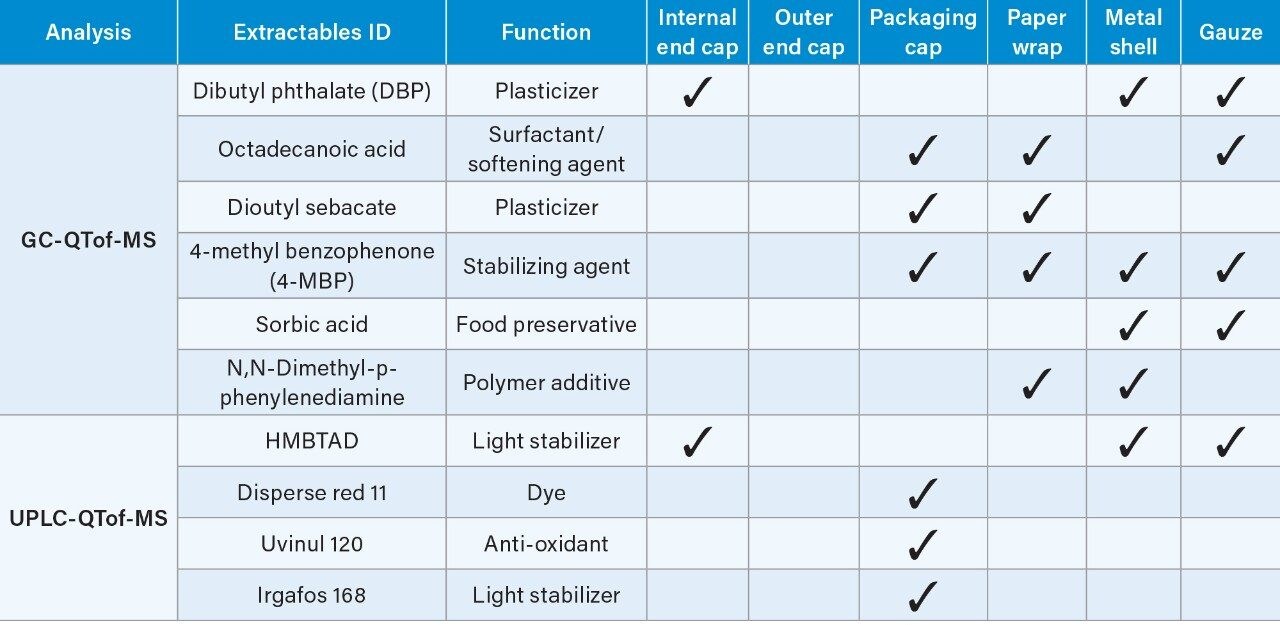
Table 1 lists the potential extractables detected in various e-cigarette component extracts analyzed by GC-QTof-MS and UPLC-QTof-MS. These compound identifications are based on the targeted match between the experimental data and the UNIFI Scientific Library for the accurate mass precursor and fragment ions, low mass error (± 5 ppm) and relatively high detector response (>1000).
Comprehensive characterization of extractables and leachables requires evaluation using multiple chromatographic techniques (UPLC and GC), multiple modes of ionization, and an integrated informatics workflow (UNIFI). Accurate mass screening using MSE data acquisition, combined with scientific libraries can be used to automatically identify target components.
UNIFI's sample comparison and elucidation toolsets are useful for quickly identifying known targets and characterizing unknown compounds. A metabolite identification workflow can be used to evaluate possible degradation or transformation products of formulation components in e-cigarette products. This study demonstrates an integrated workflow for targeted and non-targeted screening using UPLC and GC on a single MS platform with UNIFI informatics for extractable and leachable screening in e-cigarettes, food, cosmetics, and pharmaceutical packaging applications.
720006387, September 2018