This application note illustrates the transferability of ion-exchange methods across systems.
The cation exchange separation of a monoclonal antibody was developed on the ACQUITY Arc Bio System. To illustrate the ability to successfully transfer an application developed on the ACQUITY Arc Bio to another LC system, the method was transferred from the ACQUITY Arc Bio System to the Agilent 1260 Infinity Bio-inert Quaternary System. While there were minimal differences in retention time, the relative retention times across the system were within 0.02. Furthermore, the repeatability of the method was acceptable on both systems, with the ACQUITY Arc Bio System exhibiting slightly improved repeatability for both the monoclonal antibody, the C-terminal lysine variants and the smaller degradant peaks.
To evaluate proteins under native conditions, bio-inert or biocompatible systems are often preferred. These systems consist of biocompatible materials, such as MP35N (a nickel-cobalt alloy), PEEK, and titanium. The reasons for using biocompatible systems are varied, but may include: reducing corrosion from high salt concentrations, and preventing oxidation of charged species from iron ions.
One mode of chromatography often used with biocompatible systems is ion-exchange chromatography. This technique is performed to evaluate product quality of biotherapeutics, including the production of any acidic or basic impurities or charge variants through post transitional modifications. Ion-exchange separations occur under native conditions, which include aqueous mobile phases with high salt concentrations. While these are gradient methods, the analysis time may be up to one hour, with partially resolved analytes. These conditions can make method transfer challenging. To illustrate the transferability of ion-exchange methods across systems, the separation of a monoclonal antibody and its charge variants1 was transferred from an ACQUITY Arc Bio System to an Agilent 1260 Infinity Bio-inert Quaternary System.
Infliximab was obtained at 20 mg/mL. The sample was diluted in 20 mM sodium phosphate at pH 6.8 to 1 mg/mL.
|
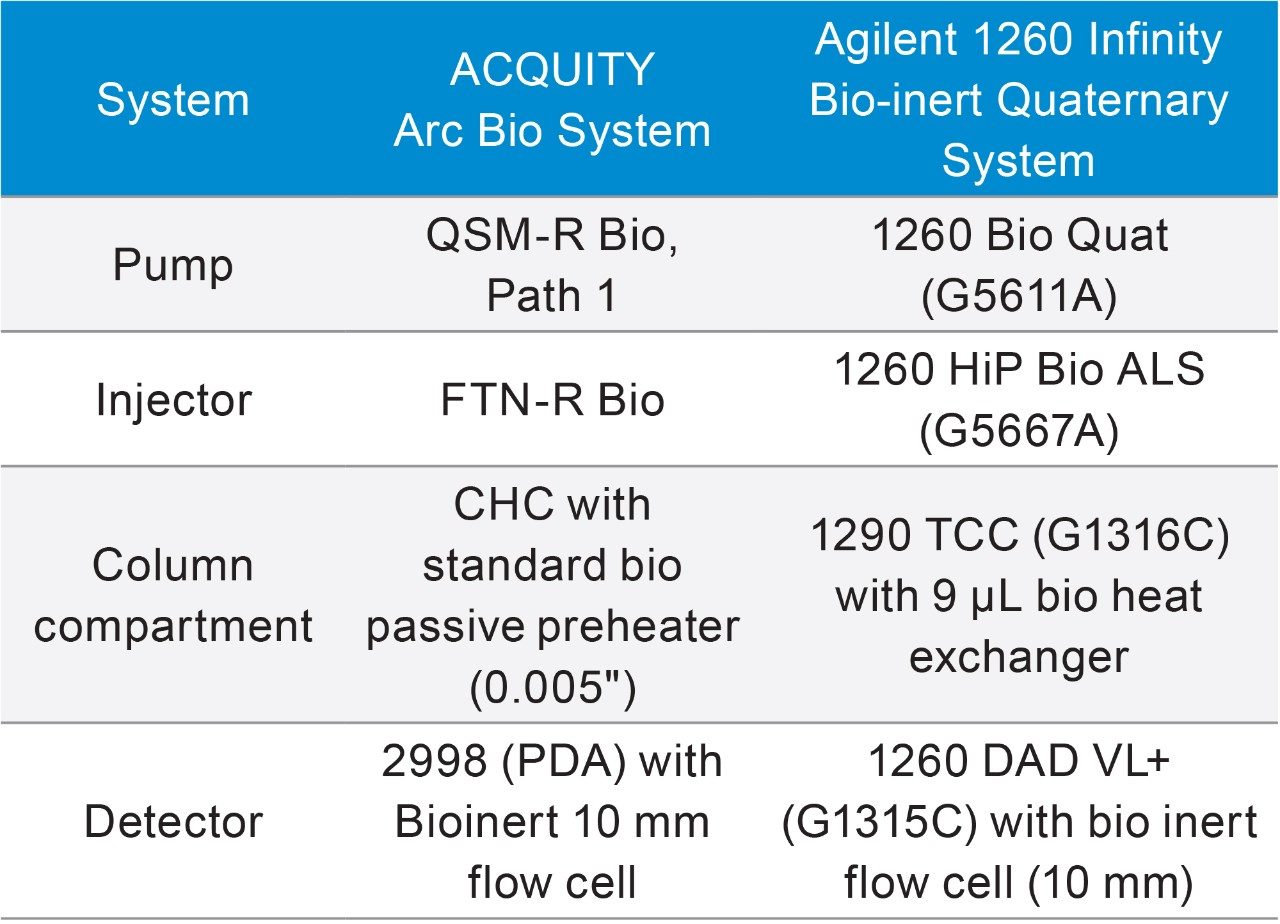
LC system: |
ACQUITY Arc Bio System and Agilent 1260 Infinity Bio-inert Quaternary System |
|
Column: |
Protein-Pak Hi Res CM, 7 μm, 4.6 × 100 mm column (p/n 186004929) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
100 mM Sodium phosphate monobasic |
|
Mobile phase B: |
100 mM Sodium phosphate dibasic |
|
Mobile phase C: |
500 mM Sodium chloride |
|
Mobile phase D: |
Water |
|
Wavelength: |
280 nm |
|
Sampling rate: |
1 Hz (ACQUITY Arc Bio) and 1.25 Hz (Agilent 1260 Bio-inert Quaternary) |
|
Filter time constant: |
Normal (ACQUITY Arc Bio) and n/a (Agilent 1260 Bio-inert ) |
|
Time |
%A |
%B |
%C |
%D |
|---|---|---|---|---|
|
Initial |
14.3 |
5.7 |
5.0 |
75 |
|
60.00 |
14.3 |
5.7 |
15.0 |
65 |
|
65.00 |
14.3 |
5.7 |
70.0 |
10 |
|
70.00 |
14.3 |
5.7 |
70.0 |
10 |
|
70.10 |
14.3 |
5.7 |
5.0 |
75.0 |
Chromatography Data Systems: Empower 3
Feature Release 3 (FR3) and OpenLAB CDS Chemstation
Data acquisition was performed using the system’s native software and processed in Empower. The Waters Data Converter was used to transfer the OpenLAB CDS Chemstation data into Empower 3 FR3.
A separation for infliximab, which contains a monoclonal antibody (IgG) and C-terminal lysine charge variants (IgGK and IgGKK), was developed using Auto●Blend Plus on an ACQUITY Arc Bio System with a cation exchange column (Protein-Pak Hi Res CM, 7 µm, 4.6 × 100 mm). The mobile phases consisted of 20 mM phosphate buffer and 25–75 mM NaCl. The 60 minute separation was optimized to separate the monoclonal antibody and its lysine variants with resolution of the acidic and basic impurities.
To ensure successful method transfer of the separation, both the originator and the receiver systems were configured to reduce system-to-system differences. For example, both the ACQUITY Arc Bio System and the Agilent 1260 Infinity Bio-inert Quaternary System used passive preheating. In addition, both systems employed diode array detectors, with collection of a single wavelength (280 nm). For comparable signal-to-noise, the sampling rate was set to 1–1.25 Hz, which was dependent on the system settings. These values were more than adequate for the peaks analyzed with peak widths typically >1 minute.
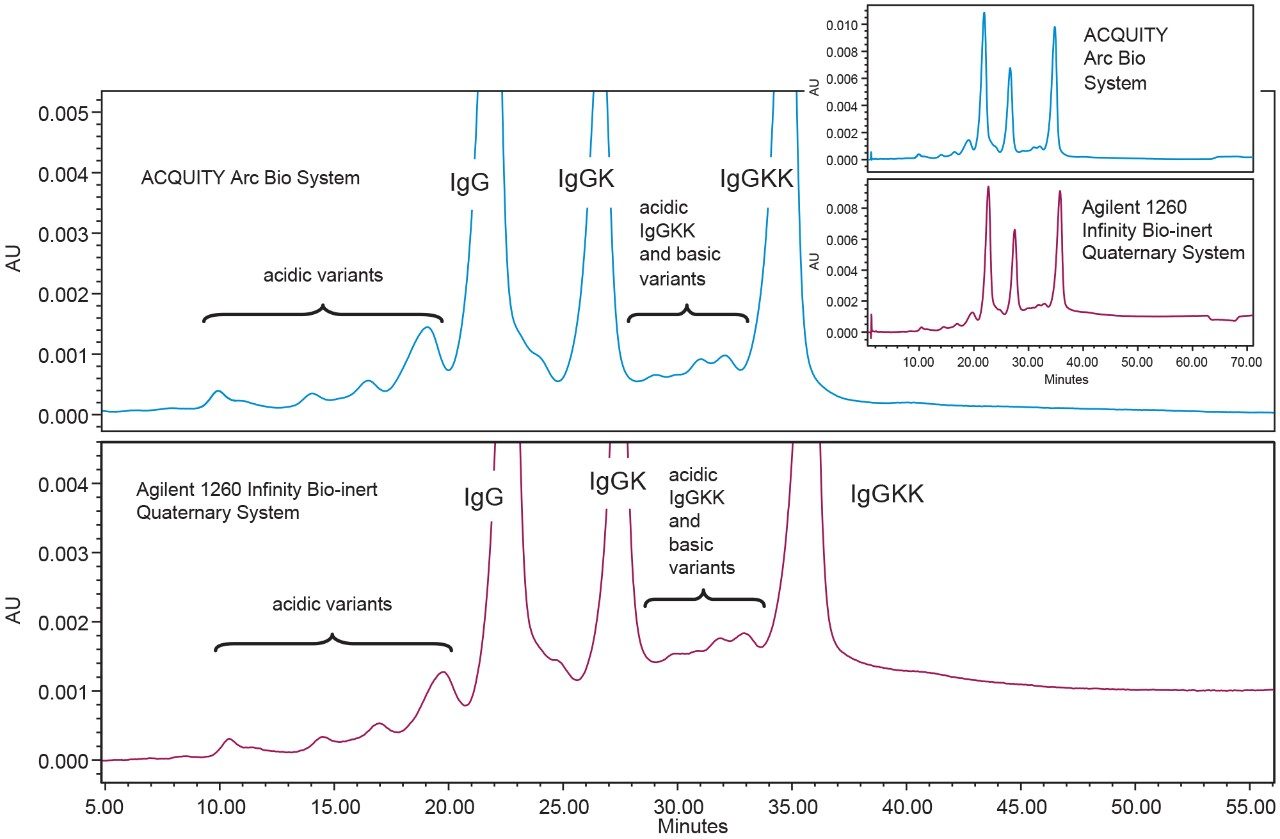
The results of the method transfer show the impact of the gradient delay or dwell volume. The dwell volume or gradient delay of each system is the volume required for the program gradient to reach the head of the column. Each system’s dwell volume was measured at 50% gradient delivery.2 Both of the quaternary systems had comparable measured dwell volumes. The ACQUITY Arc Bio System, which features Arc Multi-flow path Technology, has two flow paths in the pump, each with a different dwell volume. The ACQUITY Arc Bio System was measured at 1.32 mL for Path 1 and 1.01 mL for Path 2. In comparison, the Agilent 1260 Infinity Bio-inert Quaternary System was found to have a gradient delay of 1.57 mL. Since Path 1 was used for the separation, the ACQUITY Arc Bio System had a dwell volume approximately 0.25 mL less than the Agilent 1260 Infinity Bio-inert Quaternary System.
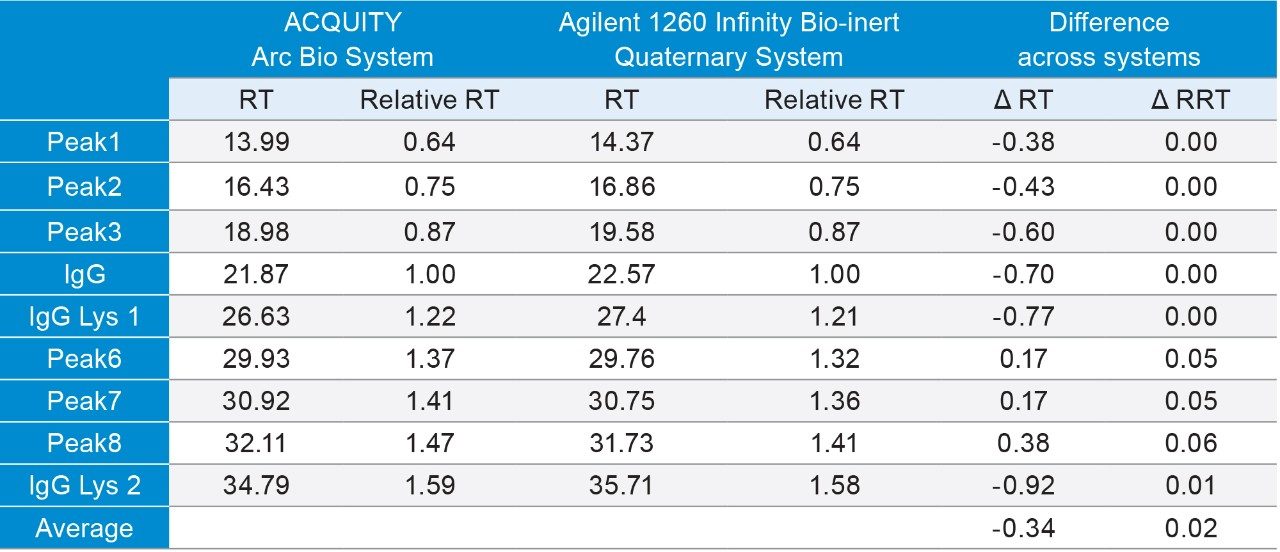
Considering this information, we can get a better understanding of the retention time shift observed across the two systems. As expected, the retention times observed on the Agilent 1260 Infinity Bio-inert Quaternary System were later than those observed on the ACQUITY Arc Bio System. While the difference in the retention times (RT) ranged from -0.92 to 0.17, the average was -0.34 min or about -0.17 mL. However, if we compare the relative retention times (RRT), using the IgG peak as the reference, we observe much closer values, with an average of 0.02 differences across the two systems. It should be worth noting that gradient delay is only one factor that can affect retention time shifts. Other variables that may affect retentively and selectivity include pre-column heating, initial equilibration from dwell volume, and mobile phase compressibility. For these reasons, relative retention time is a more reliable measure of the transferability of a method.
Method transfer can also be measured by comparing the quantitative results of an analysis. In ion-exchange chromatography, one of the primary goals of the separation is to measure the percent of acidic and basic degradants relative to the monoclonal antibody. In this case, where two C-terminal lysine charge variants of the monoclonal are also present, smaller degradant peaks were compared to the peak area of the three main peaks. The smaller variants were analyzed as two separate groups. The first consisted of the acidic variants of the IgG, which eluted before 20 minutes. The second group was comprised of the IgGKK acidic variants and the basic variants of IgG and IgGK, eluting between 28–35 minutes. Since many of these peaks are poorly resolved, to minimize variability both sets of impurities were processed in Empower 3 FR3 using the same processing method parameters.
Evaluation of the % area and the repeatability (n=3) of IgG, IgGK and IgGKK showed comparable results on both systems. Comparison of the % area across the two systems, produced values (Δ) within 0.20% for all three peaks. The measurement of the repeatability (n=3) produced values of < 1% RSD on the ACQUITY Arc Bio System and <1.2% for the Agilent 1260 Infinity Bio-inert Quaternary System for the same peaks.
Quantification of the smaller grouped peaks was more variable, in part because of the lower abundance of these peaks with % areas of <6%. For these peaks, the quantified amounts across both systems were within 0.21% area. While the %RSD’s were significantly higher on both systems, the ACQUITY Arc Bio System showed some improvement compared to the Agilent 1260 Infinity Bio-inert Quaternary System.
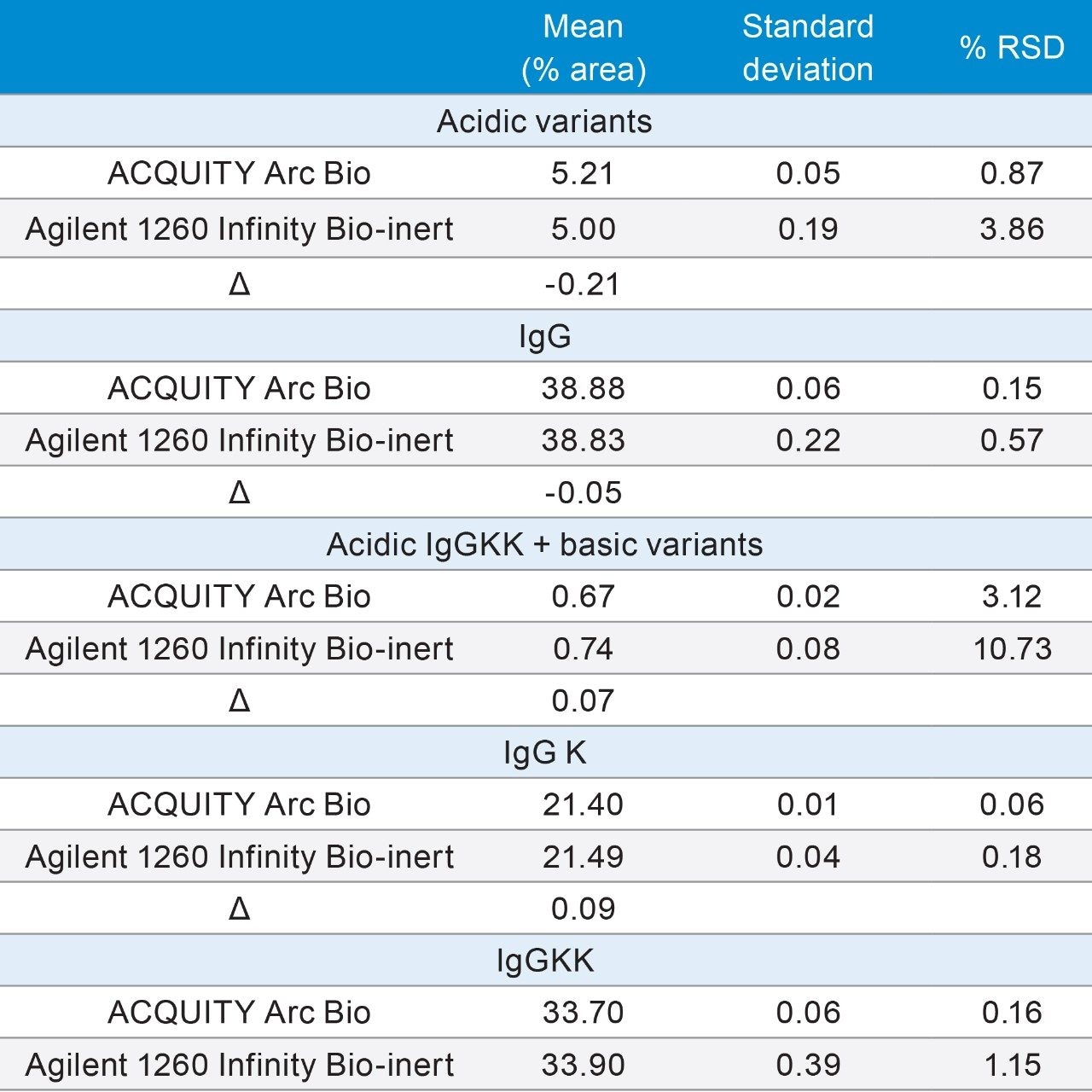
The cation exchange separation of a monoclonal antibody was developed on the ACQUITY Arc Bio System. To illustrate the ability to successfully transfer an application developed on the ACQUITY Arc Bio to another LC system, the method was transferred from the ACQUITY Arc Bio System to the Agilent 1260 Infinity Bio-inert Quaternary System. While there were minimal differences in retention time, the relative retention times across the system were within 0.02. Furthermore, the repeatability of the method was acceptable on both systems, with the ACQUITY Arc Bio System exhibiting slightly improved repeatability for both the monoclonal antibody, the C-terminal lysine variants and the smaller degradant peaks.
720006253, May 2018