
For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This technical brief demonstrates to successfully develop a 2D UPLC-MS/MS system configuration for the analysis of serum cortisol, androstenedione, and 17-hydroxyprogesterone.
Waters 2D technology provides flexibility of analysis without making changes to the system.
Sample preparation for clinical research applications of LC-MS/MS is required to reduce interferences from biological matrices, to reach the analytical sensitivity requirements and to ensure a compatible diluent is injected onto the system. Since sample preparation can be time consuming and prone to error, there is interest in simplifying the sample preparation workflow for LC-MS/MS applications.
Here we describe an approach using the Waters ACQUITY UPLC I-Class System with 2D technology to perform online sample clean-up for the analysis of steroid hormones using a Xevo TQD, following a simple protein precipitation extraction.
This system configuration is flexible, allowing the user to configure the system flow path in a number of ways that enable its operation in different modes; trapping and back transfer, heart-cutting, parallel column regeneration, at-column dilution as well as UPLC mode. Therefore, the system can be tailored to meet specific method requirements and maximize laboratory productivity. The configuration used in this technical brief utilizes a 2D UPLC trapping and back flush mode with the option to switch to direct UPLC analysis without any changes to the system.
This configuration allows for large injection volumes to maximize peak area response, where the analytes of interest are focused on Column 1 using the mobile phase gradient from the Loading BSM. The analytes are back-flushed off Column 1 onto Column 2 for chromatographic separation. While the chromatographic separation is taking place on Column 2, Column 1 is washed and re-equilibrated using the Loading BSM (Flow path shown in Figure 1).
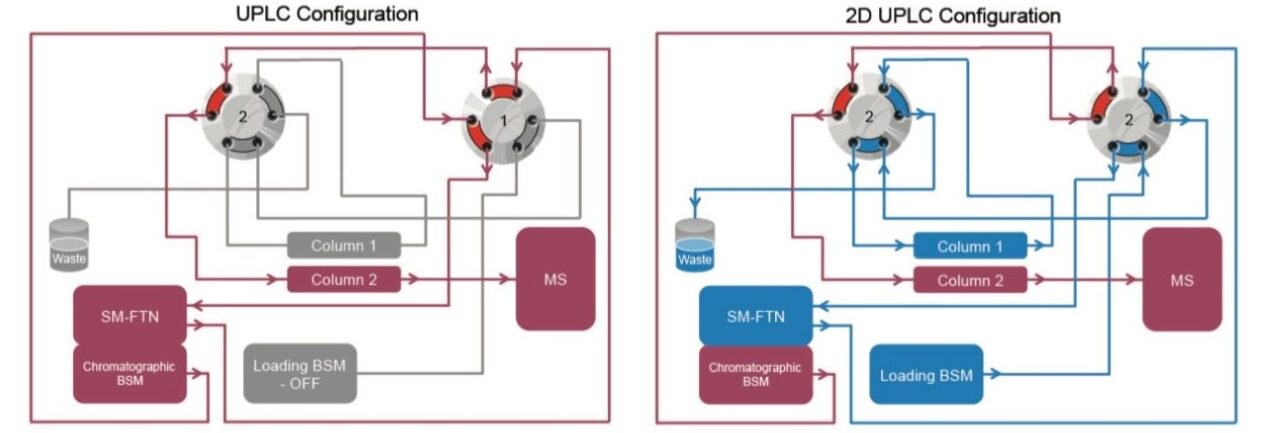
In this technology brief, a method for the analysis of serum cortisol, androstenedione, and 17-hydroxyprogesterone was successfully developed on an ACQUITY UPLC I-Class with 2D Technology with a Xevo TQD. A simple protein precipitation followed by separation using an ACQUITY UPLC BEH C18 SB VanGuard Pre-column in the first dimension and an ACQUITY UPLC HSS T3 Column in the second dimension was used to selectively clean the serum sample to provide optimum analytical performance.
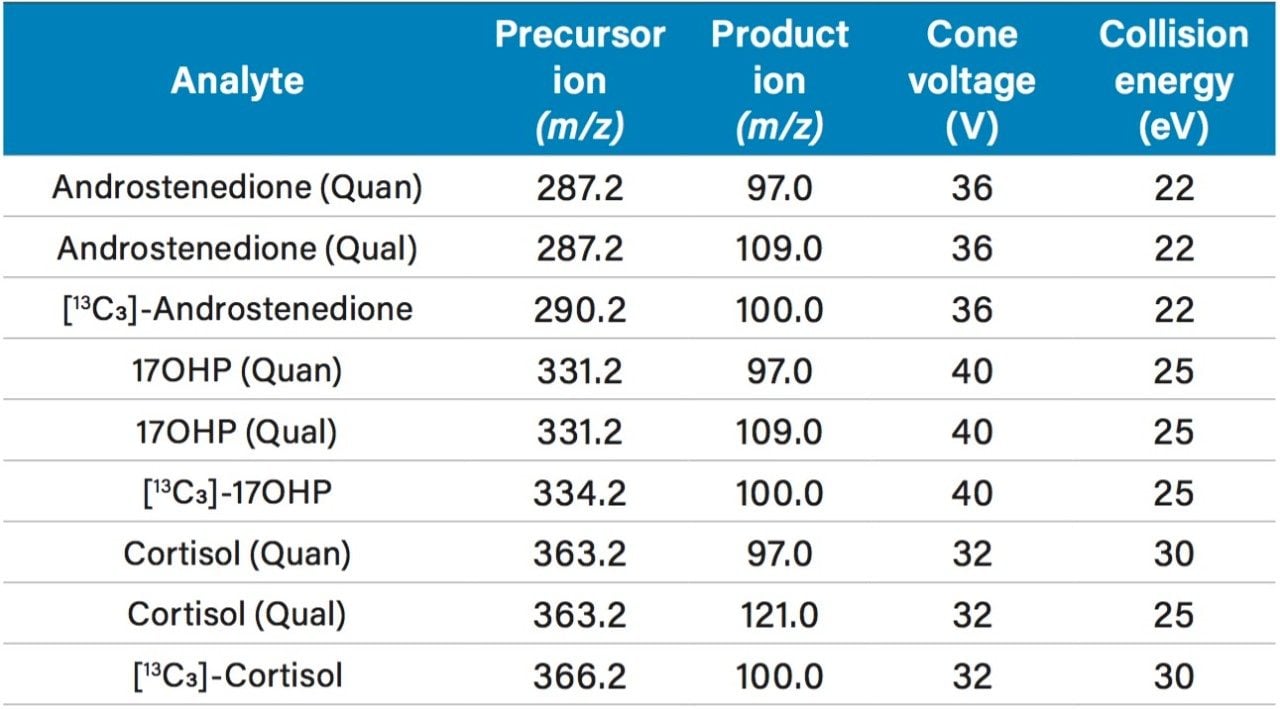
Calibrators were prepared in stripped serum over the concentration range specified in Table 1, with conversion factors provided. Calibrators, QCs and serum samples were diluted with internal standard, zinc sulfate(aq) solution and methanol, followed by centrifugation at ~25,000 g for five minutes. 100 µL of each extracted sample was injected onto the 2D UPLC system. Loading onto the first column was performed by utilizing a water/methanol/formic acid gradient on an ACQUITY UPLC BEH C18 SB VanGuard Pre-column and chromatographic separation was achieved using a water/methanol/ammonium acetate/formic acid gradient on an ACQUITY UPLC HSS T3 Column. The analytes were detected and quantified using a Xevo TQD, using electrospray in positive ionization mode and the MRM parameters are described in Table 2.
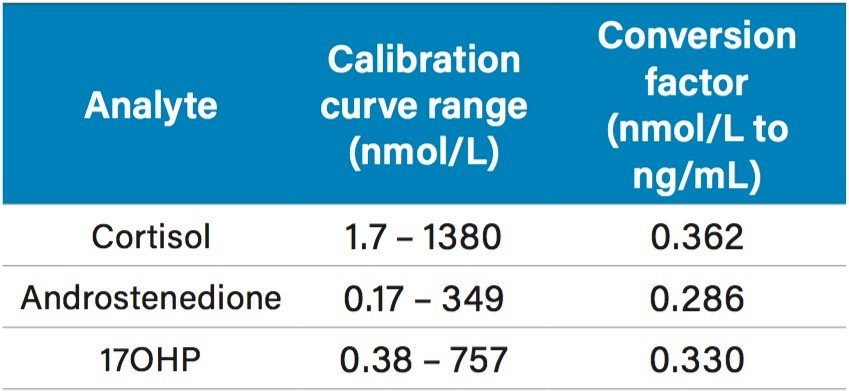
Using 2D UPLC-MS/MS, calibration curve correlation coefficients (r²) for cortisol, androstenedione, and 17-hydroxyprogesterone were >0.99 across the concentration ranges specified in Table 1.
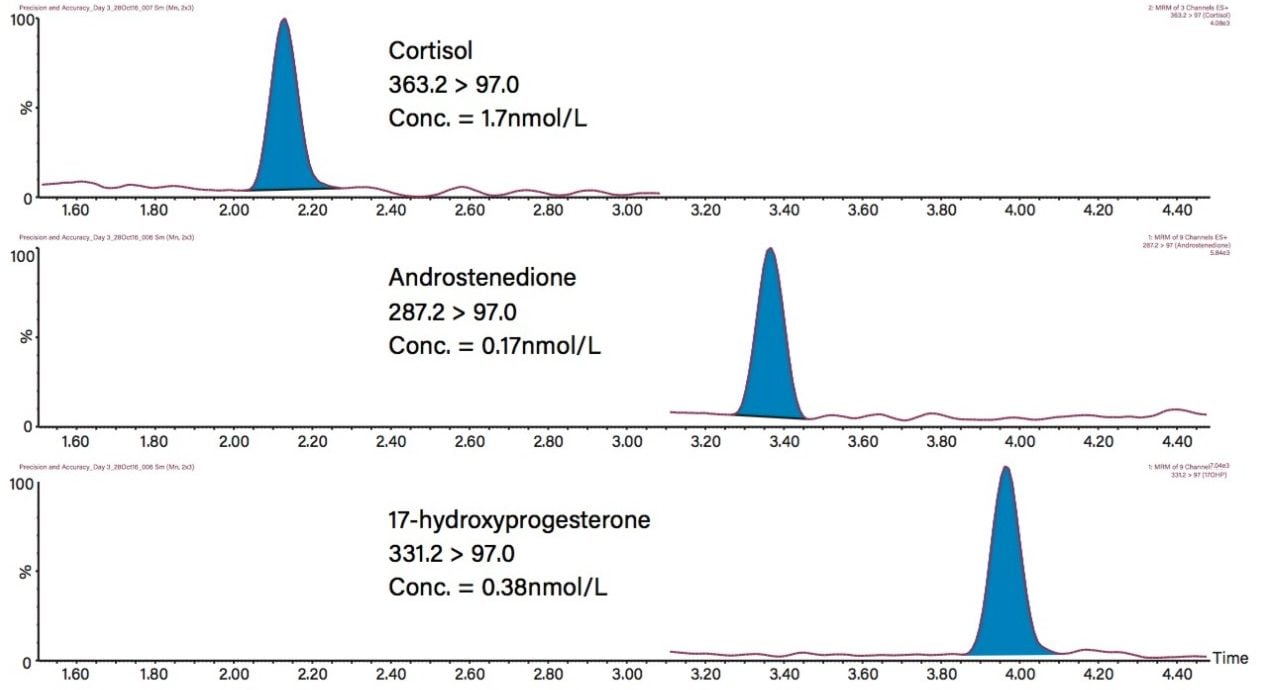
A typical chromatogram obtained from a low level calibrator can be seen in Figure 2.
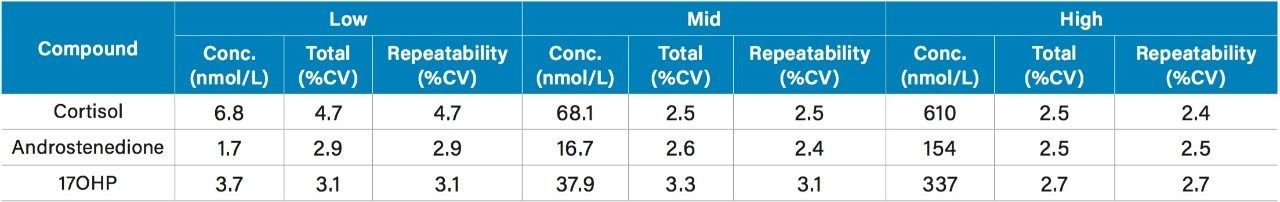
Total precision and repeatability of the method was assessed by extracting and quantifying serum samples in five replicates at low, mid, and high concentrations across five days (n=25). All results were ≤4.7% CV as shown in Table 3.
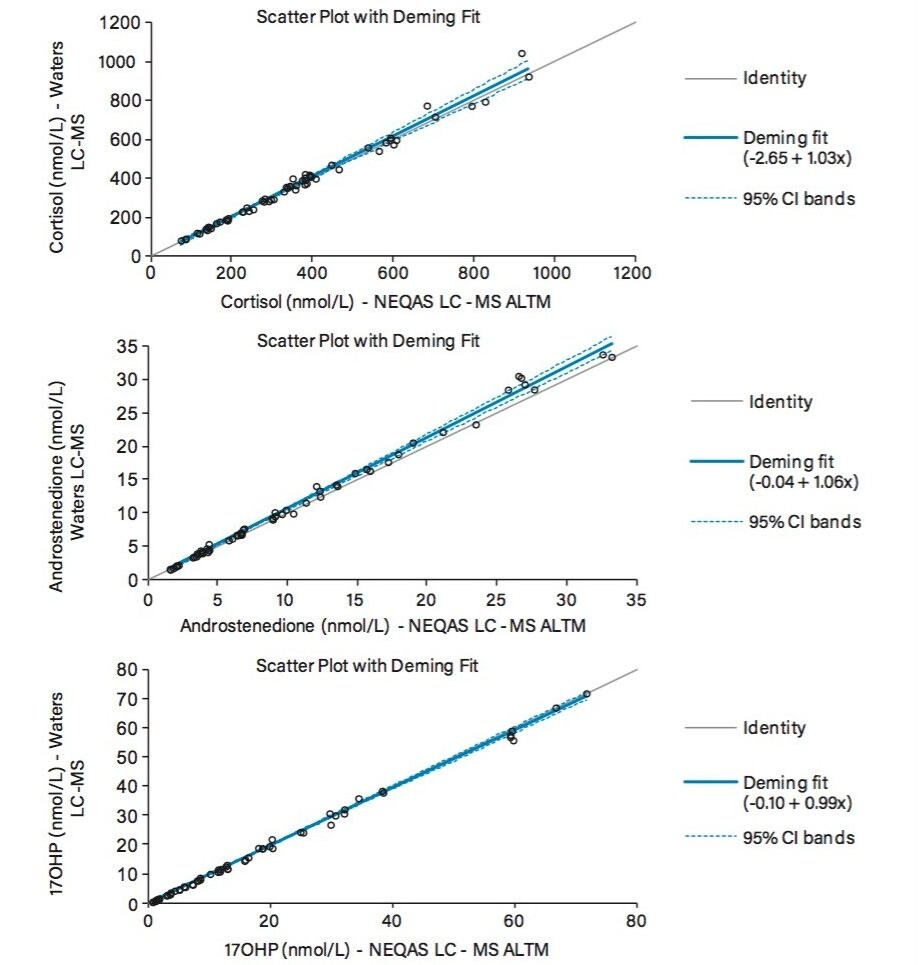
Method performance was assessed by extracting and quantifying external quality assurance samples from the UK National External Quality Assessment Service (UK NEQAS) for cortisol, androstenedione, and 17-hydroxyprogesterone over six days. Good agreement was observed when comparing the 2D UPLC-MS/MS method to the UK NEQAS LC-MS all laboratory trimmed mean (ALTM) values, having Deming regression equations of y=1.03x-2.66 for cortisol, y=1.06x–0.04 for androstenedione and y=0.99x-0.10 for 17-hydroxyprogesterone (Figure 3).
Analytical sensitivity was assessed by extracting and quantifying serum samples using 10 replicates from low to high concentrations across three days (n=30). Signal-to-noise (ptp) of >10:1 and %CV’s of <20% were obtained at 1.9nmol/L for cortisol, 0.16nmol/L for androstenedione and 0.37nmol/L for 17-hydroxyprogesterone.
A 2D UPLC-MS/MS method has been developed for the quantitative measurement of steroid hormones for clinical research. The system used is flexible and allows for switching to direct UPLC-MS/MS mode of operation with no system changes required.
The benefits of this method include:
720005964, May 2017