For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief develops a quantitative analytical method for 14 illicit or prescription drugs in preserved oral fluid.
A simple, sensitive, and quantitative UPLC-MS/MS method for substances commonly measured in oral fluid drug-testing schemes.
The analysis of illicit or prescription drugs in workplace or roadside drug-testing schemes has become an important aspect of forensic toxicology. The use of oral fluid as an alternate matrix for these tests has increased in popularity over the last decade due to a number of reasons. Oral fluid collection is a non-invasive technique and it can be achieved without the privacy and adulteration issues associated with urine collection. In contrast to blood samples, oral fluid does not require medically-trained staff to collect the sample. Unlike urine, oral fluid can be more indicative of current impairment or intoxication. The Quantisal Oral Fluid Collection Device (Immunalysis, USA) allows 1 mL of sample to be collected into a stabilizing buffer which promotes stability of the sample during transportation to the testing laboratory.
A simple solid-phase extraction is used to eliminate matrix effects that result from additives and stabilizers in oral fluid collection devices. Combining the Waters ACQUITY UPLC I-Class System with the Xevo TQD allows these compounds to be detected at levels lower than the currently recommended maximum cut-offs for confirmation assays in workplace drug-testing schemes.1 This UPLC-MS/MS combination also permits a compound-specific quantitative determination of the relevant analytes.
Oral fluid samples were collected and preserved using the Quantisal Oral Fluid Collection Device according to the manufacturer’s directions. It is generally understood that the collected oral fluid is diluted by a factor of four once it has been added to the buffer in the device, and the concentrations stated in this technical brief relate to those in neat oral fluid. Once collected, the samples were stored at 4 °C for at least 24 hours prior to analysis.
Ten microlitres (1.125 ng) of deuterated internal standard (ISTD) mixture was added to 350 μL preserved oral fluid (either calibrator or quality control samples) and the sample was diluted with 4% phosphoric acid (350 μL). The samples were extracted by solid-phase extraction (SPE) using a modified version of Danaceau et al: 2 the wells in a 96-well Waters Oasis MCX μElution plate (P/N 186001830BA) were conditioned with 200 μL methanol followed by 200 μL 18.2 MΩ water. The entire diluted sample was added to each well. After loading, the wells were washed with 200 μL 2% formic acid followed by 200 μL 50% methanol. After drying under vacuum for 10 min, all samples were eluted with 200 μL acetonitrile/propan-2-ol (60:40, v/v) containing 5% ammonium hydroxide. The samples were evaporated to dryness under a stream of nitrogen at 50 °C (for a maximum of 15 min) and reconstituted in 87.5 μL water/acetonitrile (95:5, v/v). The collection plate was covered with a silicone/PTFE treated cap mat and vortex-mixed for 1 min. Five microlitres were analyzed by UPLC-MS/MS.
|
System: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC BEH C18, 130Å, 1.7 μm, 2.1 mm x 100 mm (P/N 186002352) |
|
Column temp.: |
40 °C |
|
Flow rate: |
0.40 mL/min |
|
Mobile phase A: |
0.1% formic acid |
|
Mobile phase B: |
acetonitrile |
|
Wash solvent: |
acetonitrile/water (95:5, v/v) |
|
Purge solvent: |
0.1% formic acid |
|
Time(min) |
%B |
Curve |
|---|---|---|
|
0 |
2 |
Initial |
|
1.50 |
13 |
6 |
|
1.80 |
13 |
6 |
|
2.65 |
36 |
6 |
|
3.00 |
36 |
6 |
|
3.40 |
50 |
6 |
|
4.25 |
95 |
6 |
|
4.75 |
95 |
6 |
|
4.80 |
2 |
6 |
|
MS system: |
Xevo TQD |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
1.0 KV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas: |
800 L/Hr |
|
Cone gas: |
20 L/Hr |
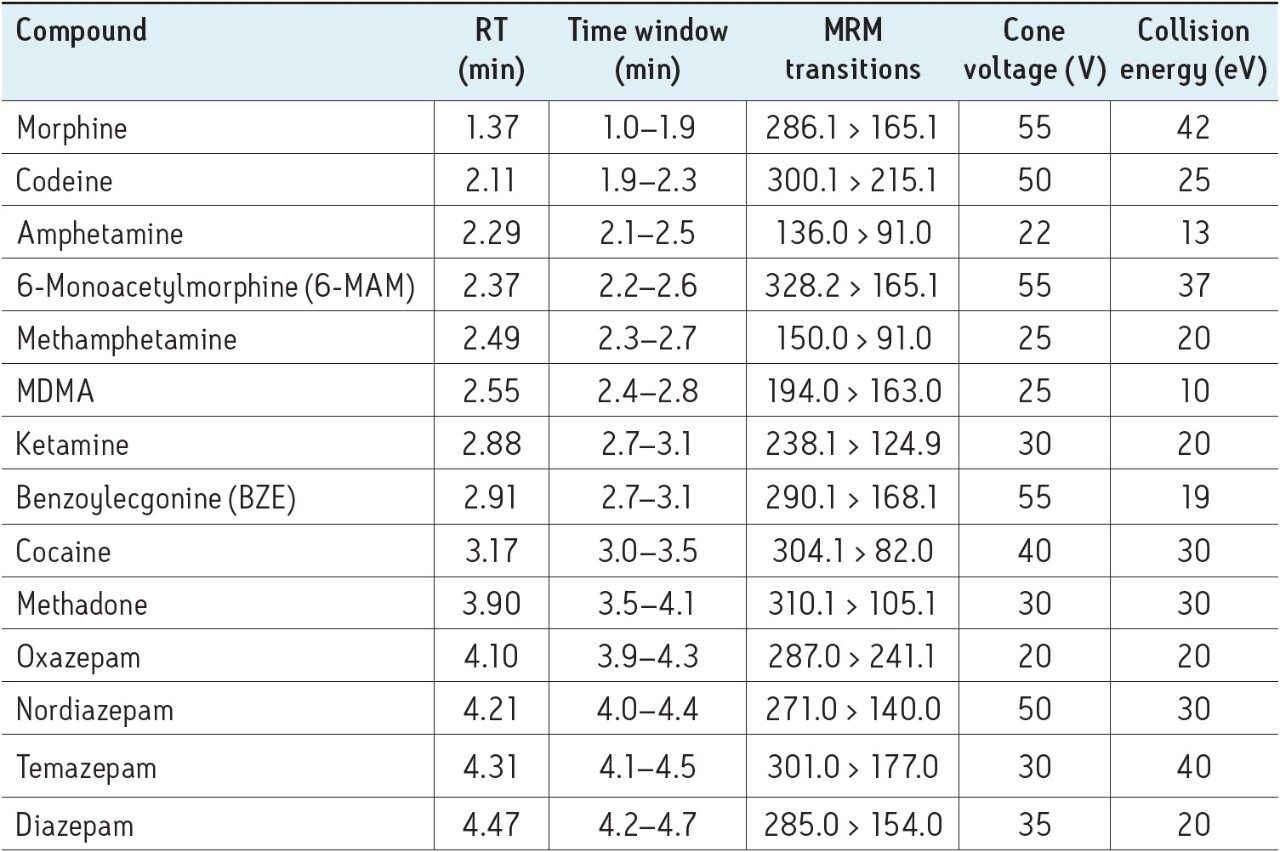
The acceptance criteria for a positive identification of analytes were: the retention time to be within 0.2 min of predicted and the quantifier/qualifier ion ratio to be within 20% of the predicted ratio, which was based on the average of the ratios across the entire calibrator range.
To investigate linearity for all analytes, spiked preserved oral fluid calibrators and quality control (QC) samples were prepared daily (the concentrations in neat oral fluid ranged from 0 ng/mL to 500 ng/mL) and analyzed on four different days. Peak areas for each MRM trace were generated automatically using the TargetLynx Application Manager and referenced to the appropriate ISTD peak area. Quantitative calibration curves were plotted using a 1/x weighting with a quadratic fit applied to all analytes. Interday coefficient of determination (assessed over four days) was >0.995 for each analyte.
The limit of detection (LOD) was defined as the lowest concentration which gave a signal-to-noise ratio >10:1 (for both transitions) in spiked preserved oral fluid. The lower limit of quantitation (LLOQ) was defined as the lowest concentration which gave a signal to noise ratio >10:1 (for both transitions) and ion ratios within 20% of expected and the achieved concentration was within 20% of target with a %RSD of <20% in preserved oral fluid over the four day period. The LOD and LLOQ for each analyte are summarised in Table 2 along with the concentration of the lowest QC sample assayed. Extraction recovery and matrix effects for each analyte were investigated in six different sources of preserved oral fluid at three concentrations (5, 25, and 100 ng/mL), with the ISTDs at 12.5 ng/mL. The mean % recovery for each analyte was matched by that of the appropriate deuterated internal standard and was acceptable for this assay. The matrix effects were matched by the appropriate deuterated internal standard and were shown to be less than 25% for the majority of analytes with the %RSD less than 15% for all analytes.
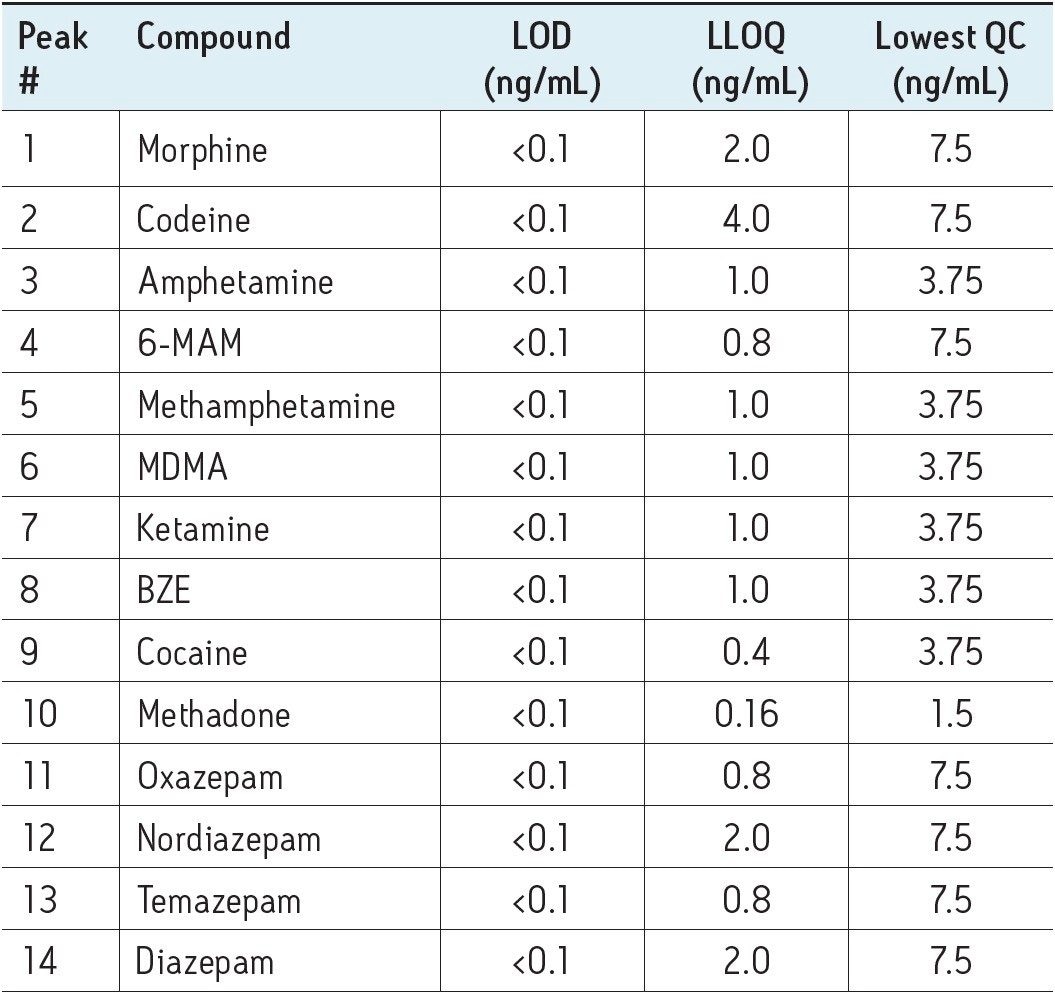
Interday accuracy and precision were assessed by analyzing three QC samples at low, medium, and high concentrations over four different days. The mean achieved values for the quality control replicates over the four day period at the three concentration levels were within 15% of target and the % RSD was <10%.
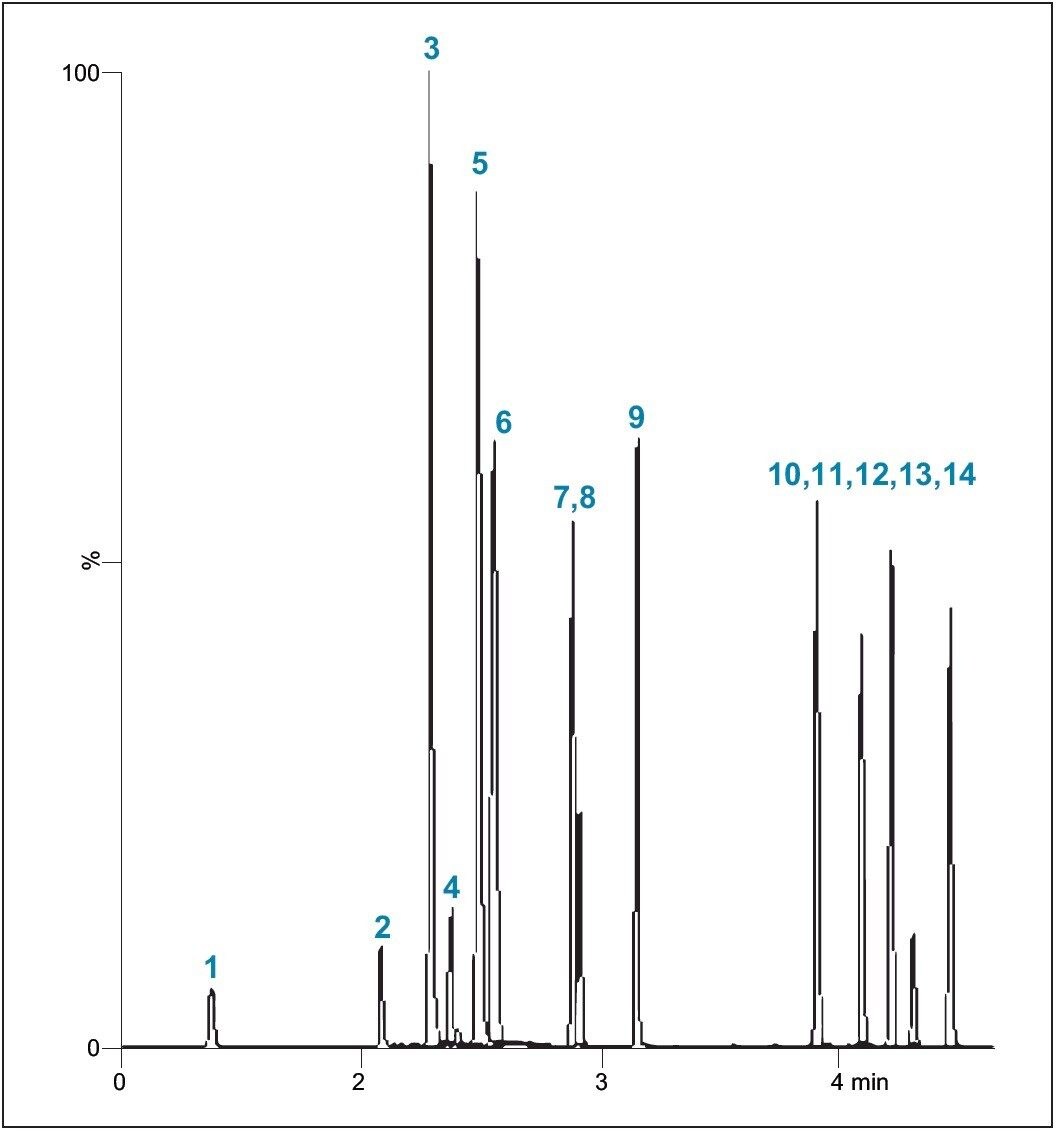
Figure 1 shows a chromatogram of a processed oral fluid QC sample at the lowest level assayed.
The rise of workplace and roadside drug testing has highlighted the need for a quick, accurate, reliable, and robust method to quantify both illicit and prescribed drugs in various biological matrices. The use of preserved oral fluid allows for simple, supervised, and non-invasive collection of a matrix which contains analytes commonly measured in such testing schemes.
The use of the Waters ACQUITY UPLC I-Class System allows for a quick and robust analytical method that can detect the analytes in a single run. The demonstrated injection-to-injection time of 7 min, combined with the simple sample preparation method utilizing Oasis MCX plates, minimizes matrix effects from the stabilizers used in commercial collection devices. This allows for high sample throughput. Furthermore the superior sensitivity of the Xevo TQD permits detection of the analytes at levels lower than the currently recommended maximum cut-offs for confirmation assays in workplace drug testing.
This is a proof of principle demonstration of an analytical method, which may include examples of typical results that can be achieved with the stated configuration. This method represents a basic starting point from which users should perform their own in-house validation.
720005584, March 2016