
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to modernize and demonstrate improvements for the USP impurity method for cetirizine hydrochloride tablets using a column packed with 2.5 μm particles.
Most USP HPLC methods commonly used to analyze raw materials and finished products were developed and validated using older columns technologies, often containing 5 μm particles. By using modern column technologies and USP-allowed method changes, the methods can be made much less time consuming while still meeting all the criteria listed in the monograph.
USP methods are commonly used to analyze raw materials and finished products. Most of the USP HPLC methods were developed and validated using older columns technologies, often containing 5 μm particles. By using modern column technologies and USP-allowed method changes, such as smaller particle sizes and shorter columns, the methods can be made much less time consuming while still meeting all the criteria listed in the monograph.
Columns packed with smaller particles can be used to increase productivity without compromising the quality of a separation. When the ratio of column length to particle size is held constant, columns packed with smaller particles give the same separation efficiency in a shorter time. Combined with higher optimum linear velocities, columns containing smaller particles can substantially reduce analysis times.
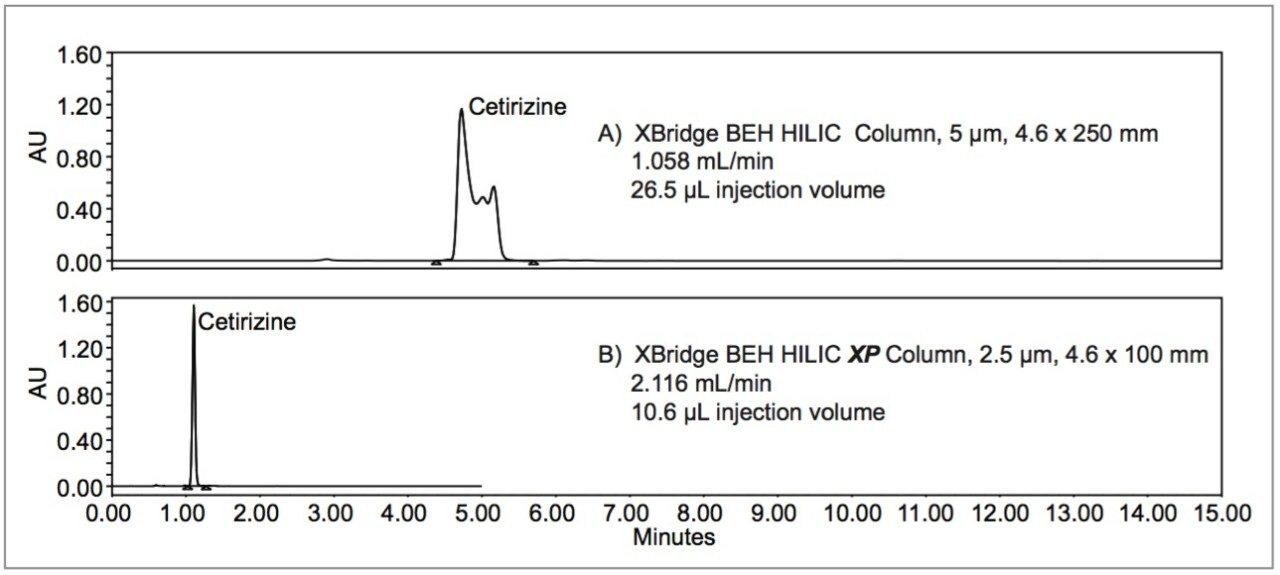
The USP method for the analysis of organic impurities in cetirizine hydrochloride calls for a 4.0 x 250 mm, 5 μm, L3 column. L3 columns are classified as containing porous silica particles, 1.5 – 10 μm in diameter, or a monolithic rod. The method was scaled to use a 4.6 x 250 mm XBridge BEH HILIC Column (p/n 186004454) packed with 5 μm particles. The separation is isocratic, with the mobile phase containing acetonitrile (93%) and an acidified aqueous solution of tetrabutylammonium hydrogen sulfate (7%). The injection volume was geometrically scaled using the Waters Column Calculator from the monograph value of 20.0 μL to 26.5 μL. Unexpectedly, the chromatogram obtained showed a distorted peak for cetirizine (see Figure 1A).
The method was then scaled down to a 4.6 x 100 mm, XBridge BEH HILIC XP Column (p/n 186006087) packed with 2.5 μm particles. The injection volume was geometrically scaled to 10.6 μL and the flow rate was scaled to 2.116 mL/min. The cetirizine peak obtained using these chromatographic conditions was not distorted. As shown in Figure 1B, the new conditions produce a desirable Gaussian peak, meeting the system suitability criteria of USP tailing NMT 2.0 and peak area relative standard deviation (RSD) NMT 10.0%. A tailing factor of 1.3 and an RSD of 0.8% were obtained. The run time was reduced from 15 minutes to 3 minutes.
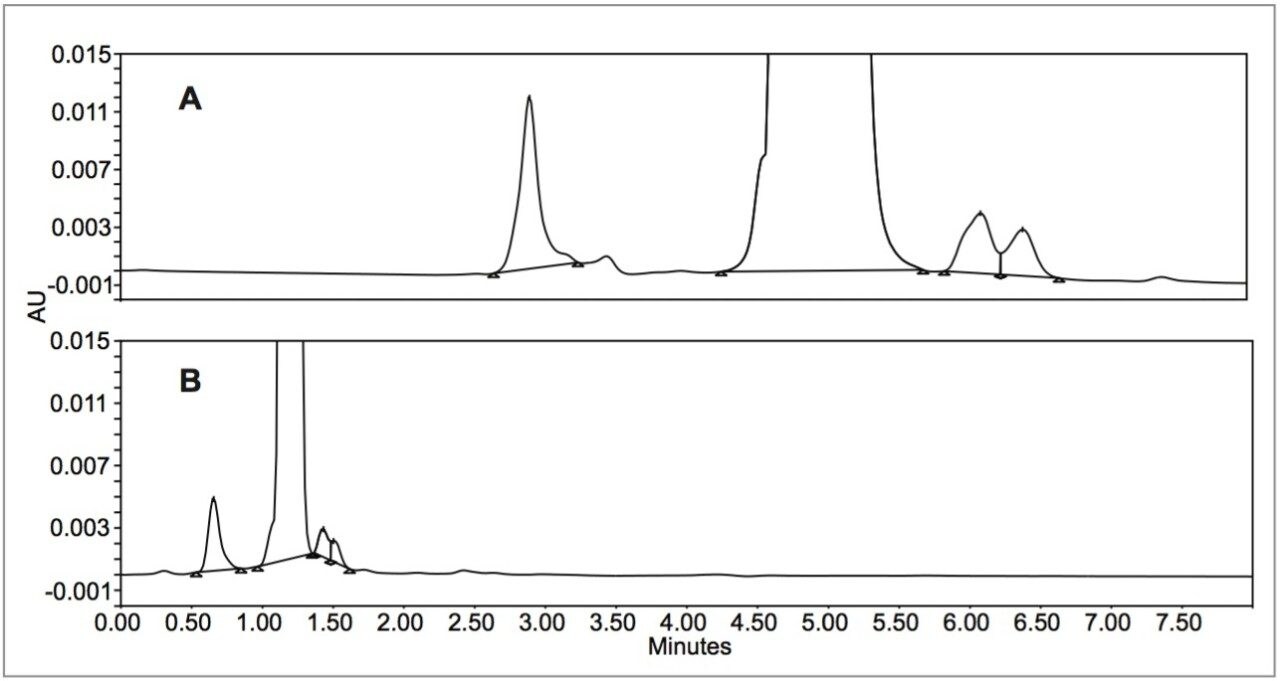
Upon close examination of the prescribed preparation of sample and mobile phase, the distorted peak shape observed on the larger, 5 μm column is due to a mismatch between the sample diluent and the mobile phase. The diluent contains a higher water concentration (9%), no buffer, and a slightly different pH than the mobile phase. The peak becomes more regular when the injection volume is lowered, but at the cost of sensitivity for low level impurities. By using a smaller column packed with smaller particles, the injection volume is scaled down (proportional to the column volume) and the peak shape improves. Even though a smaller volume injection is made, the sensitivity for all peaks is maintained as the smaller column produces narrower peaks (see Figure 2).
Updating USP methods using newer column technology can decrease run times while still meeting all the specifications of USP <621> guidelines. By using columns containing 2.5 μm particles, faster methods can be developed for use with existing HPLC instrumentation. Here we have demonstrated an unexpected advantage which is gained by modernizing the USP impurity method for cetirizine HCl tablets. Not only was the analysis time reduced by a factor of five, but an issue with peak distortion was avoided.
720005825, November 2016