
A major concern of many laboratory managers and analytical chemists is the speed at which they can process samples. Ballistic methods are useful when analysis speed is important. They can improve sample throughput and turnaround times for both routine sample analyses and during regular performance monitoring. Ballistic methods can be created easily from simple pressure/flow measurements and some trial injections with a few different normalized gradient slopes.
A major concern of many laboratory managers and analytical chemists is the speed at which they can process samples. A high analysis speed can be used in two separate ways depending on the focus of the analyst. Increased speed can be used to raise the number of samples processed over a certain amount of time (sample throughput), or to obtain faster answers for a limited number of samples (sample turnaround). In both cases, increasing the speed of analysis would be beneficial and one way to gain analysis speed is to employ ballistic methods.
Increasing sample turnaround is especially important for routine system1 performance checks. Every chromatographic system should have its performance checked regularly to ensure data quality and reduce potential downtime.2 These checks can be performed using ballistic methods, thereby reducing analysis time. This permits analysts to quickly establish confidence in their system and then return to running their samples. Rapidly analyzing a standard, such as a Waters UPC2 Quality Control (QC) Reference Material, makes performing routine system checks a more efficient tool in laboratories.
This application note focuses on the creation and use of gradient ballistic methods. It is illustrated with the UPC2 QC Reference Material on different stationary phase particle sizes and column dimensions.
|
ACQUITY UPC2 conditions |
|
|---|---|
|
Instrument: |
ACQUITY UPC2 with CM-30S Column Manager |
|
Columns: |
ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 2.1 mm x 50 mm (p/n 186007457) ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 2.1 mm x 150 mm (p/n 186007458) ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 3 mm x 50 mm (p/n 186007459) ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 3 mm x 150 mm (p/n 186007460) ACQUITY UPC2 Torus 2-PIC Column, 130A, 1.7 μm, 3 mm x 50 mm (p/n 186007600) ACQUITY UPC2 Torus 2-PIC Column, 130A, 1.7 μm, 3 mm x 75 mm (p/n 186007601) ACQUITY UPC2 Torus 2-PIC Column, 130A, 1.7 μm, 3 mm x 100 mm (p/n 186007602) ACQUITY UPC2 Torus 2-PIC Column, 130A, 1.7 μm, 3 mm x 150 mm (p/n 186007603) |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Methanol |
|
Equilibration: |
5 column volumes (CVs) at gradient start %B (“Equil” in Table 1) |
|
Gradient: |
3% to 60% B for Trefoil Columns and 5% to 60% B for Torus Columns (see below for calculation of gradient slope and gradient time, “Tg” in Table 1) |
|
Hold: |
3 column volumes (CVs) at gradient end %B (“Hold” in Table 1) |
|
Flow rate: |
Ballistic flow rates varied based on column dimensions and particle sizes (“F” in Table 1) |
|
Column temp.: |
40 °C |
|
ABPR setting: |
1,500 psi 2,000 psi (ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 3 mm x 50 mm only) |
|
Detection (UV): |
240 nm (PDA) |
|
Injection volume: |
Injection volume varied based on column dimensions (see figures below) |
|
Data management: |
Empower 3 CDS |
A vial of UPC2 QC Reference Material (p/n 186007950) was placed onto the instrument for injection.
The term “ballistic” method has been used to describe a chromatographic method giving much faster analysis speed than “conventional” methods.3 Since “ballistic only” describes a result relative to a “conventional” reference point, the term can have different meanings. For this application note, we are interested in gradient ballistic methods. Here, a ballistic method uses a high flow rate appropriate for the system and a steep normalized gradient slope, appropriate for the analytes of interest. The ballistic flow rate is a flow rate producing approximately 80–85% of the instrument pressure limit when the mobile phase is most viscous. The ballistic gradient slope is determined as described below. We chose this definition because it is a general approach for obtaining the fastest analysis speed from any chromatographic instrument, column, and sample.
Conventional chromatographic methods commonly use a van Deemter plot4 derived optimal flow rate for the column, in order to gain the highest efficiency possible. Ballistic methods using an elevated flow rate and gradient slope potentially sacrifice some column efficiency to gain increased analysis speed. The column efficiency cost of this ballistic method trade-off depends on the factors that affect the shape of the van Deemter plot for the column that is used. Use of columns packed with smaller particle stationary phases mitigate this cost as does the use of a gradient vs. an isocratic method. In supercritical fluid chromatography (SFC), the use of low viscosity/high diffusivity CO2 in the mobile phase also reduces this trade off cost.
The ballistic method can be created by the following sequence:
1. Determine the ballistic flow rate for the system and conditions used.
a. Select the column (plus any desired guard), mobile phases, gradient range to use, the column temperature, and any other conditions that can affect the system pressure.
b. Determine the mobile phase composition in the desired gradient range that gives the maximum back pressure.5
c. Isocratically flow at this mobile phase composition on the chromatographic instrument equipped with the selected column under the selected conditions. Adjust the flow rate to achieve a system pressure in the range of 80–85% of the instrument pressure limit. This is the ballistic flow rate for the system and conditions used.
2. Scout different normalized gradient slopes for the analytes of interest.
a. At the experimentally determined ballistic flow rate, select normalized gradient slopes (defined in the example below) to try with the analytes of interest. Calculate the corresponding gradient times (see Eq. 4 below). If appropriate, add a post-gradient hold at high strong solvent percent to elute highly retained compounds.
b. Create and run methods for each trial normalized gradient slope with the analytes of interest.
3. Determine the ballistic method.
a. Select the method with the steepest normalized gradient slope at the ballistic flow rate that gives acceptable chromatographic performance (e.g. resolution of critical pairs, elutes all the peaks, etc.).
Ballistic methods – ACQUITY UPC2 example
The ACQUITY UPC2 System currently has an instrument pressure limit of 6,000 psi at a maximum flow rate of 3.3 mL/min. We therefore selected a system pressure target of 5,000 psi + 200 psi (ca. 83% of this pressure limit) for determining a ballistic flow rate. This gave as fast a chromatographic speed and as comparable a fluid density as is practical across the various columns and methods.
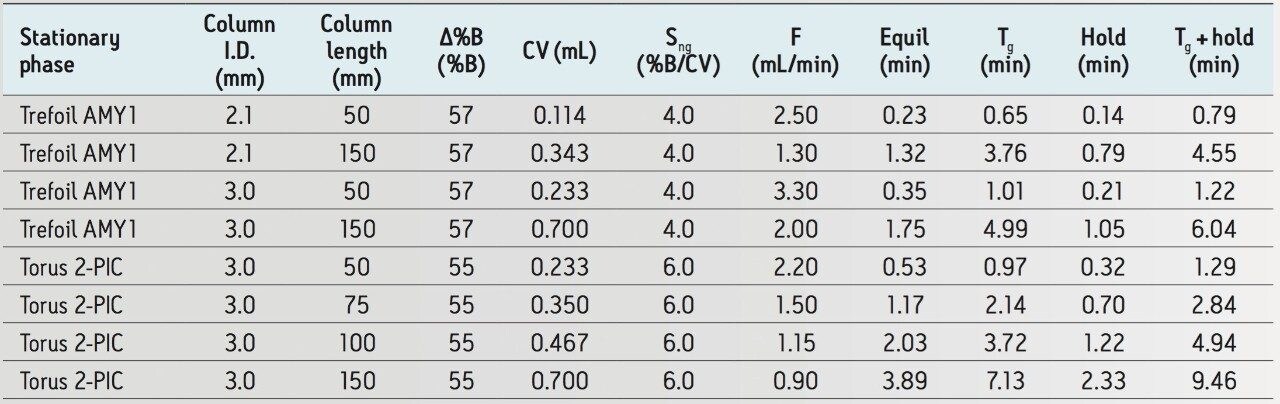
We started with the minimum automatic backpressure regulator (ABPR) setting of 1,500 psi to allow the fastest flow rates and therefore the shortest run times. With the above gradients, the mobile phase will have a maximum viscosity at 60% B. To determine the ballistic flow rate for each column, a mobile phase composition of 60% B was run through each column (at the above conditions) on the instrument with an initial flow rate of 1 mL/min. The instrument pressure was monitored and the flow was adjusted to determine which flow rate would give the desired system pressure target for each column. The resulting ballistic flow rates are summarized in Table 1 for each column. Note that with the ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 3 mm x 50 mm (p/n 186007459), the flow rate of 3.3 mL/min was reached before the system pressure target could be achieved. In this case, we increased the ABPR setting (see above conditions) to attain the system pressure target.
When creating chromatographic gradient methods that are equivalent across various column geometries, it is convenient to calculate and use the same normalized gradient slope. This takes into account changes in both the column geometry and flow rate. The normalized gradient slope, Sng, is expressed as the ratio of the change in percent mobile phase B (Δ%B) during the gradient and the number of mobile phase column volumes (#CVs) passed through the column during the gradient, Eq. 1. The units of Sng are %B/CV.
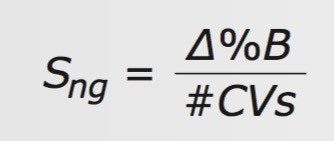
The #CVs is calculated from the gradient time (Tg), flow rate (F), and CV for the column used, Eq. 2. The CV for each of the above columns was calculated using the ACQUITY UPLC Columns Calculator6 and is summarized in Table 1.
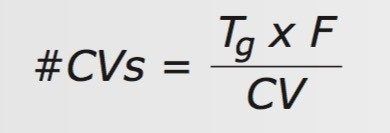
These equations are combined to give the normalized gradient slope equation as a function of those parameters, Eq. 3.
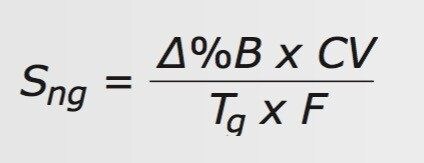
Rearranging Eq. 3 to solve for Tg gives Eq. 4.
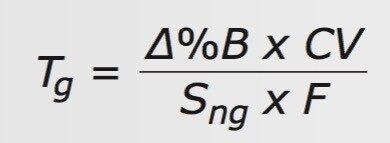
By experimenting with different Sng values (2, 4, 6 %B/CV), we found that the Torus Columns gave good chromatography and speed with the UPC2 QC Reference Material (p/n 186007950) using Sng = 6.0 %B/CV (see Figure 2). For the Trefoil Columns, a more shallow, and therefore slower, Sng = 4.0 %B/CV was needed to provide sufficient separation of the racemic trans-stilbene oxide, 1 (see Figure 3). Table 1 summarizes the parameters used for each column (see conditions above for particle sizes).
The type of ballistic methods described here relies on the instrument and column reproducibly generating approximately the same resistance to solvent flow. Waters chromatographic instruments of a given configuration satisfy this requirement as does the reproducibility of Waters columns. However, different instrument configurations or deteriorating column health can alter flow resistance. The former may require changes in the ballistic method to adapt to (e.g. a different type of column manager, detector, etc). The latter can be mitigated by use of guard cartridges (must be present when the ballistic methods are created) or better sample clean up.
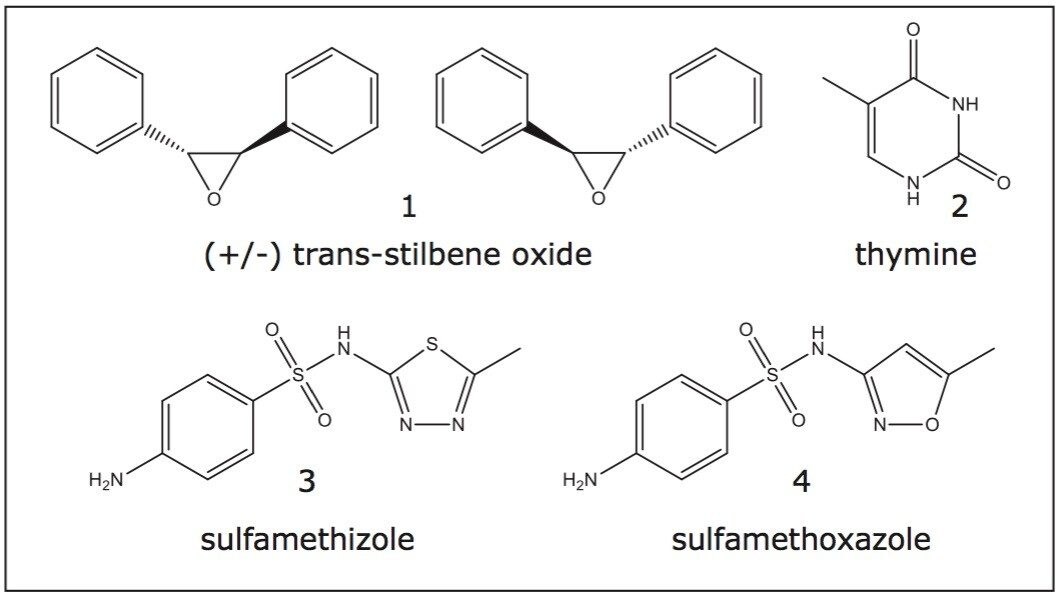
To illustrate the use of ballistic methods, the UPC2 QC Reference Material was analyzed on an ACQUITY UPC2 System. Figure 1 shows the compounds present in the UPC2 QC Reference Material. The presence of the racemic compound trans-stilbene oxide, 1, allows this standard to be used on both chiral and achiral UPC2 Columns. Additionally, the presence of the sulfamethizole, 3, and sulfamethoxazole, 4, allow for mass spectrometry detection. This standard is ideal for multiple instrument configurations, including UPC2-UV/MS.
Figures 2 and 3 show the chromatograms obtained when using the ballistic methods from Table 1, created for the ACQUITY UPC2 Torus 2-PIC Columns and the ACQUITY UPC2 Trefoil AMY1 Columns in the listed geometries.
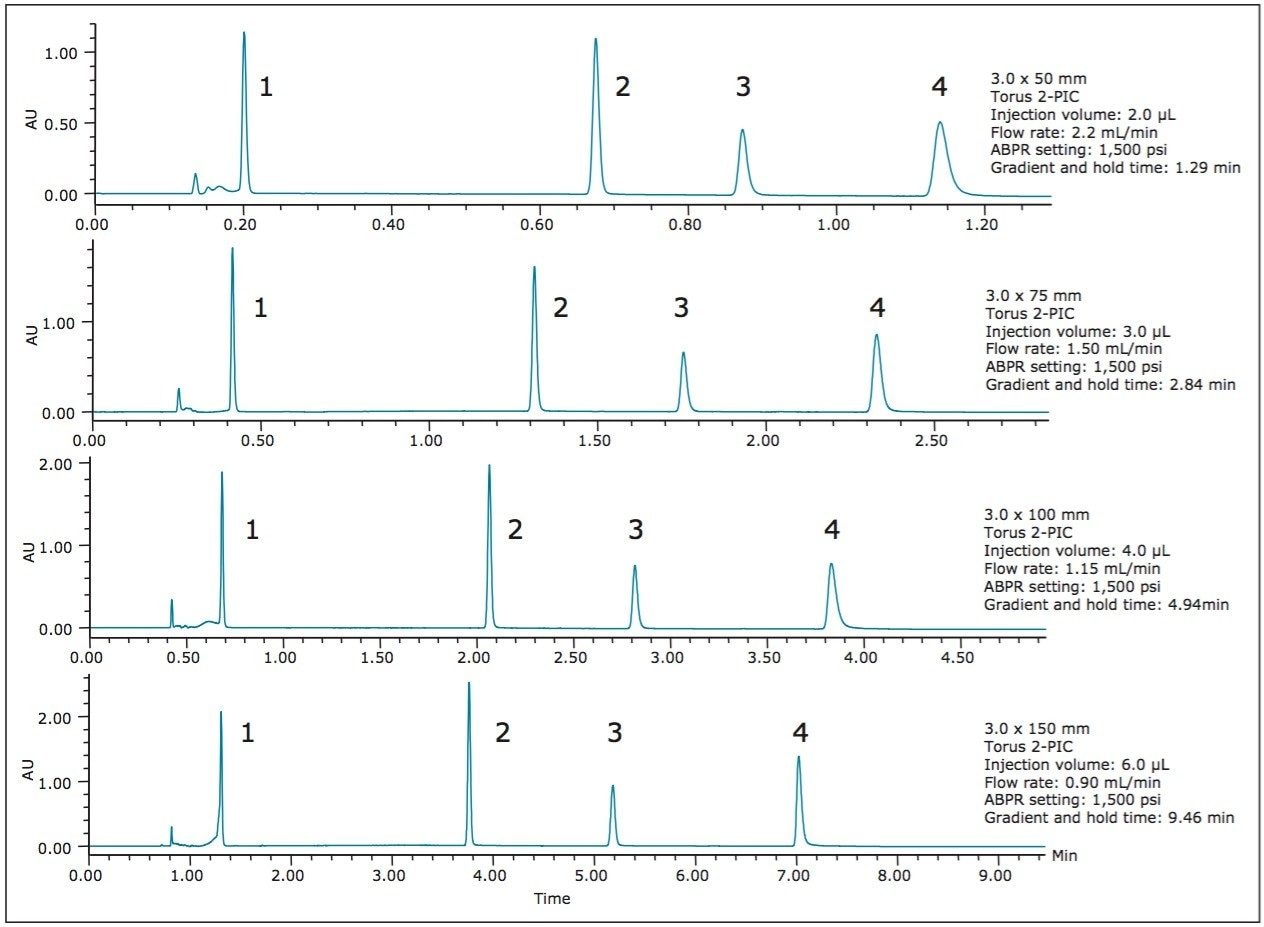
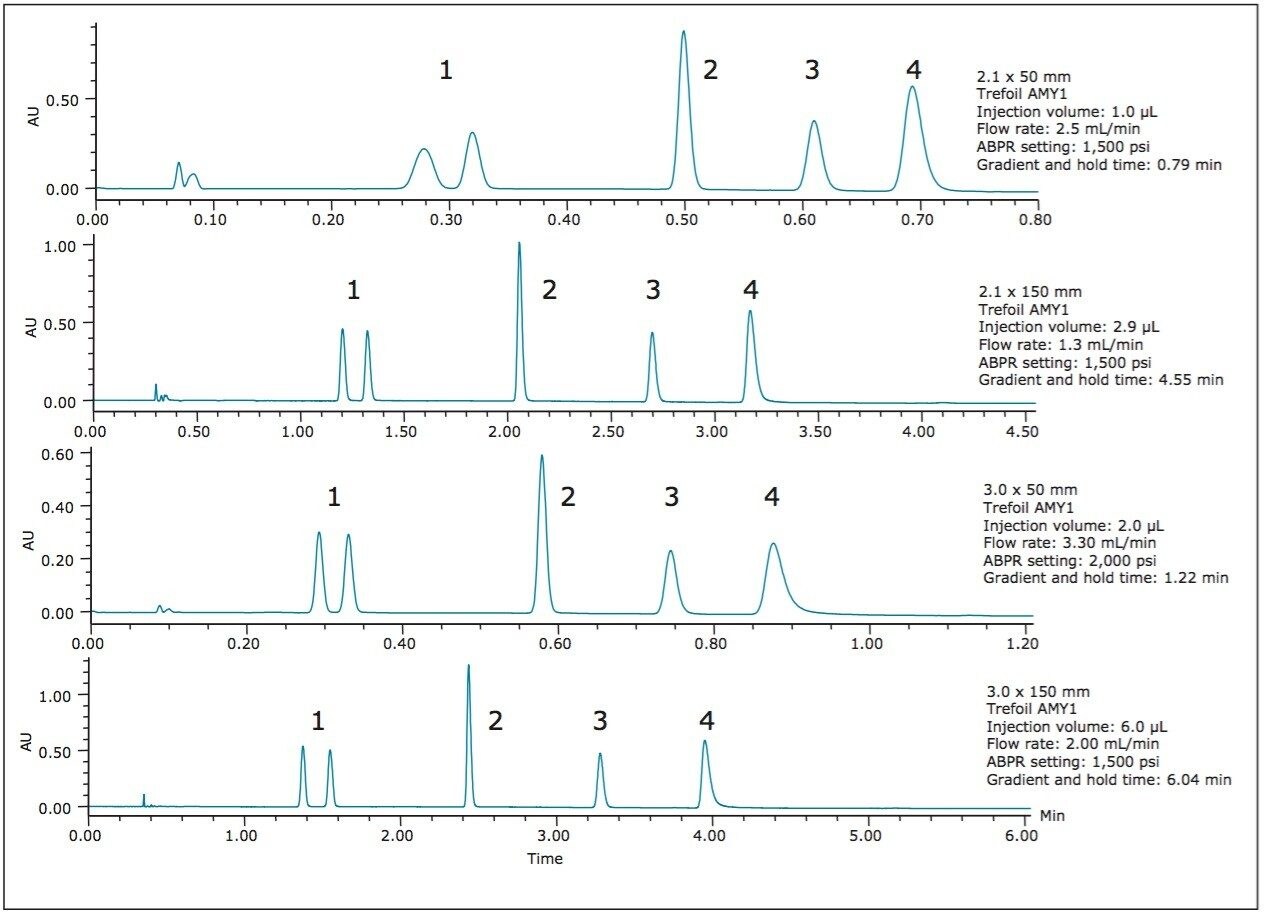
The UPC2 QC Reference Material components are well separated on the various Torus and Trefoil Column configurations. The ballistic method run times in this example vary widely from 0.79 min with the ACQUITY UPC2 Trefoil AMY1 Column, 2.5 μm, 2.1 mm x 50 mm (p/n 186007457) to 9.46 min for the ACQUITY UPC2 Torus 2-PIC Column, 130Å, 1.7 μm, 3 mm x 150 mm (p/n 186007603). In all cases, the run times are as short as possible, given the selected conditions, the instrument pressure limit, and the normalized gradient slopes.
Ballistic methods are useful when analysis speed is important. They can improve sample throughput and turnaround times for both routine sample analyses and during regular performance monitoring. Ballistic methods can be created easily from simple pressure/flow measurements and some trial injections with a few different normalized gradient slopes.
Combining ballistic methods with the convenient and easy-to-use UPC2 QC Reference Material allows analysts to spend the minimum time necessary confirming proper system operation before running their valuable samples. This gives greater confidence in the experimental results generated.
720005555, January 2016