This application note describes the fast, sensitive quantification of infliximab from rat plasma using the ProteinWorks eXpress Digest Kit and Protocol.
The ProteinWorks eXpress Digest Kit was successfully used to purify infliximab from a typical set of standard curve and QC samples in rat plasma. A limit of quantification of 10 ng/mL was readily achieved, while maintaining excellent linearity and single digit precision.
As more drug development efforts focus on large molecules such as antibodies or ADC’s, traditional “small molecule” scientists find themselves challenged not only by the complexity and time consuming nature but also the multitude of potential workflows that exist for protein quantification by LC-MS. This is also true for researchers investigating protein biomarkers where the use of ELISA’s and other immuno-affinity (IA) methods are commonplace. While IA methods are sensitive and simple to execute, poor reagent reproducibility,
lack of standardization, cross-reactivity, limited linear dynamic range, and other short-comings have led the drive to convert to LC-MS, especially for discovery and early development/pre-clinical studies. LC-MS workflows, however, encompass a multitude of sub-segments, each having many steps. Decisions about specific reagents, as well as the time, temperature, and concentration of the reagents or steps can all affect sensitivity, making it difficult to quickly arrive at a method which produces the desired detection limits. This application note describes the fast, sensitive quantification of infliximab (Figure 1) from rat plasma using the ProteinWorks eXpress Digest Kit and Protocol. Using a single universal sample prep method with pre-weighed, lot-traceable reagents and a set of carefully developed, yet generic set of simple step-wise instructions, an LLOQ of 10 ng/mL infliximab was achieved.
Infliximab was first immuno-purified from 35 µL rat plasma using a 96-well Protein A agarose-based plate. Samples were then prepared for LC-MS analysis using the ProteinWorks eXpress Digest Kit and Protocol. Finally, signature peptides were cleaned-up using the ProteinWorks µElution SPE Clean-up Kit and Protocol.
|
LC system: |
ACQUITY UPLC |
|
Detection: |
Xevo TQ-S Mass Spectrometer, ESI+ |
|
Column: |
ACQUITY UPLC Peptide BEH C18, 300Å, 1.7 μm, 2.1 mm x 150 mm |
|
Column temp.: |
55 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Data management: |
MassLynx (v4.1) |
|
Flow rate (mL/min) |
Time (min) |
Profile %A |
Profile % B |
Curve |
|---|---|---|---|---|
|
0.3 |
0.0 |
100.0 |
0.0 |
6.0 |
|
0.3 |
1.0 |
100.0 |
0.0 |
6.0 |
|
0.3 |
7.0 |
50.0 |
50.0 |
6.0 |
|
0.3 |
8.0 |
10.0 |
90.0 |
6.0 |
|
Capillary (kv): |
3.0 |
|
Cone (V): |
30.0 |
|
Source offset (V): |
50.0 |
|
Source temp. (°C): |
150.0 |
|
Desolvation temp. (°C): |
600.0 |
|
Cone gas flow (L/hr): |
150.0 |
|
Collision gas flow (mL/min): |
0.15 |
|
Nebulizer gas flow (Bar): |
7.0 |
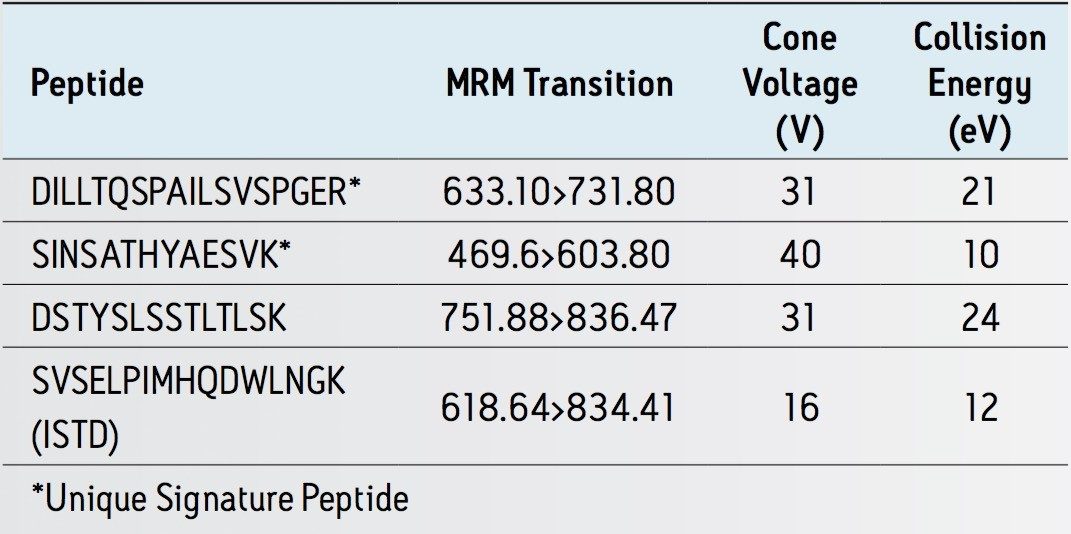
With the infliximab US patent expiration date of 2017 drawing ever closer,1 the focus on this important drug in CRO’s as well as biosimilar research labs has increased. However, typical workflows are incredibly complex, with a multitude of choices and options. This makes the development of high sensitivity methods challenging. In this application note, we have used the ProteinWorks eXpress Digest Kit to simplify and streamline the process. Infliximab samples were affinity purified, digested, and peptides extracted using SPE in under 6 hours total. This enabled data to begin to be acquired the same day, with several 96-well plates being run by the following morning. Multiple unique signature peptides as well as a generic human peptide were simultaneously monitored for use in quantification. The best sensitivity was achieved using the unique peptide SINSATHYAESVK from the heavy chain, while additional unique (DILLTQSPAILSVSPGER, light chain) and generic (DSTYSLSSTLTLSK, light chain) infliximab peptides were monitored for confirmation. A unique peptide (SVSELPIMHQDWLNGK) from a common murine mAb standard (p/n 186006552 ) was used as the internal standard.
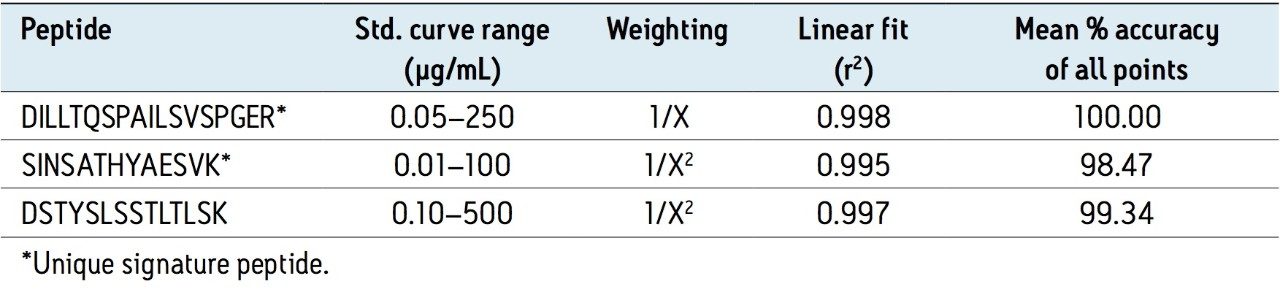
Using the optimized protocol and reagents provided in the kit, only 35 μL of plasma was needed to achieve a detection limit of 10 ng/mL for infliximab (Figure 2). Linearity and accuracy of the standard curves arising from each peptide are summarized in Table 2. The primary, and most sensitive quantitative peptide, SINSATHYAESVK, was linear over 4 orders of magnitude with a mean accuracy of >98% for all points on the curve. The additional two peptides were linear over 3.5 orders of magnitude with average accuracies >99% for all curve points.
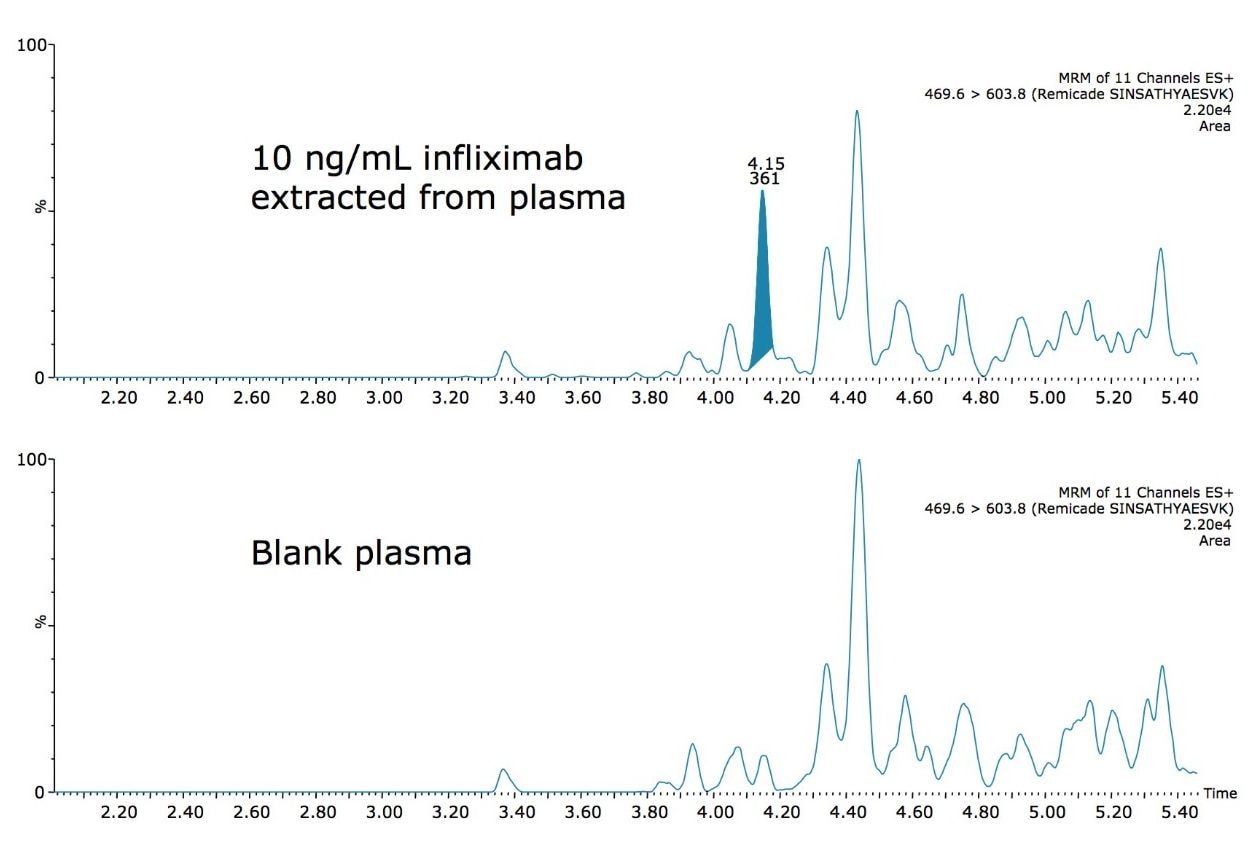
In addition, the accuracy and precision for the QC samples was excellent with %CVs all <6%. This is summarized in Table 3. In fact, the average %CV for QC samples from the SINSATHYAESVK peptide was <3%.
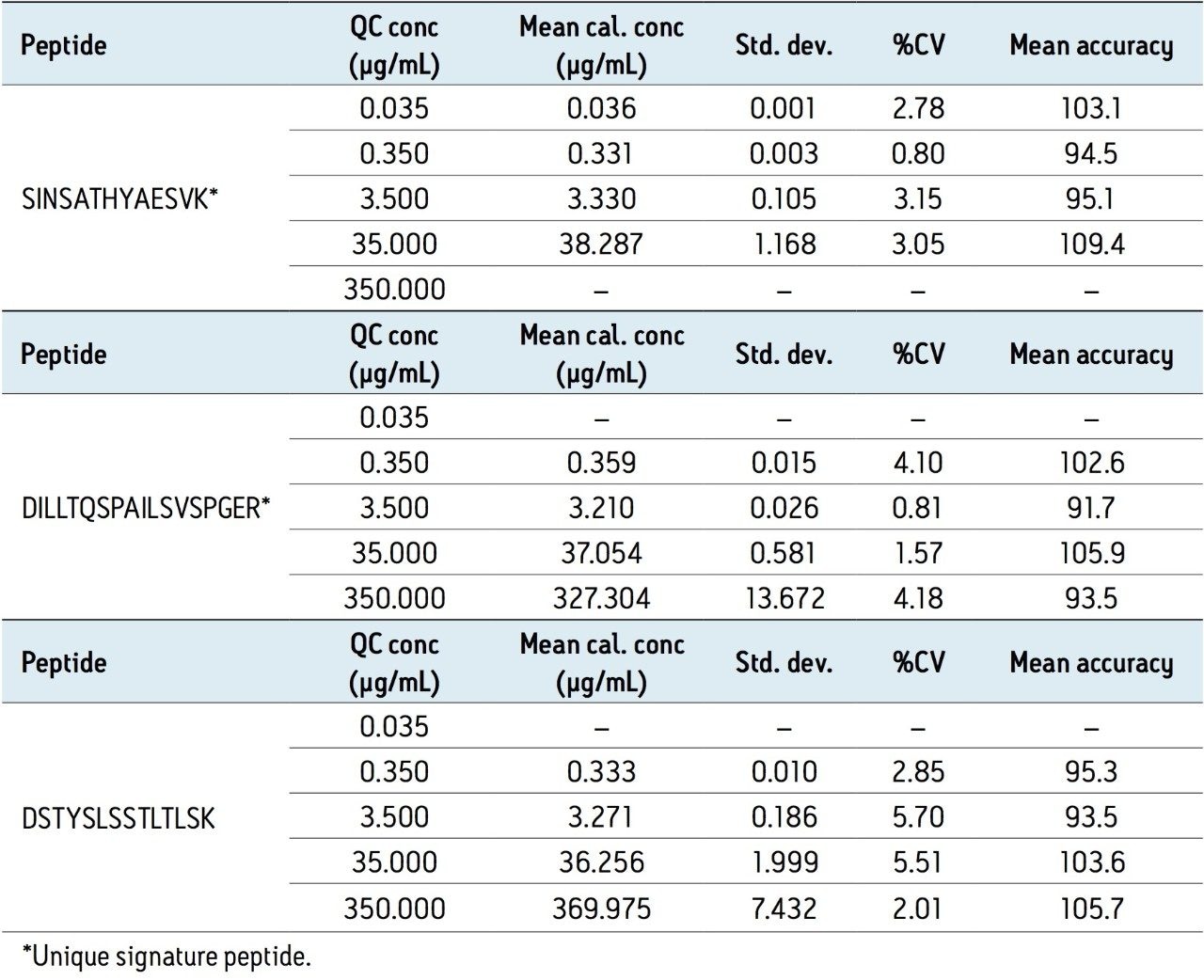
From an assessment of the chromatographic data, it is clear that the quality of the data in terms of peak width and separation from residual endogenous components facilitated both the low level detection and the very high accuracy and precision that were achieved. This can be observed and is highlighted in the QC chromatograms from all signature peptides in Figures 3–5.
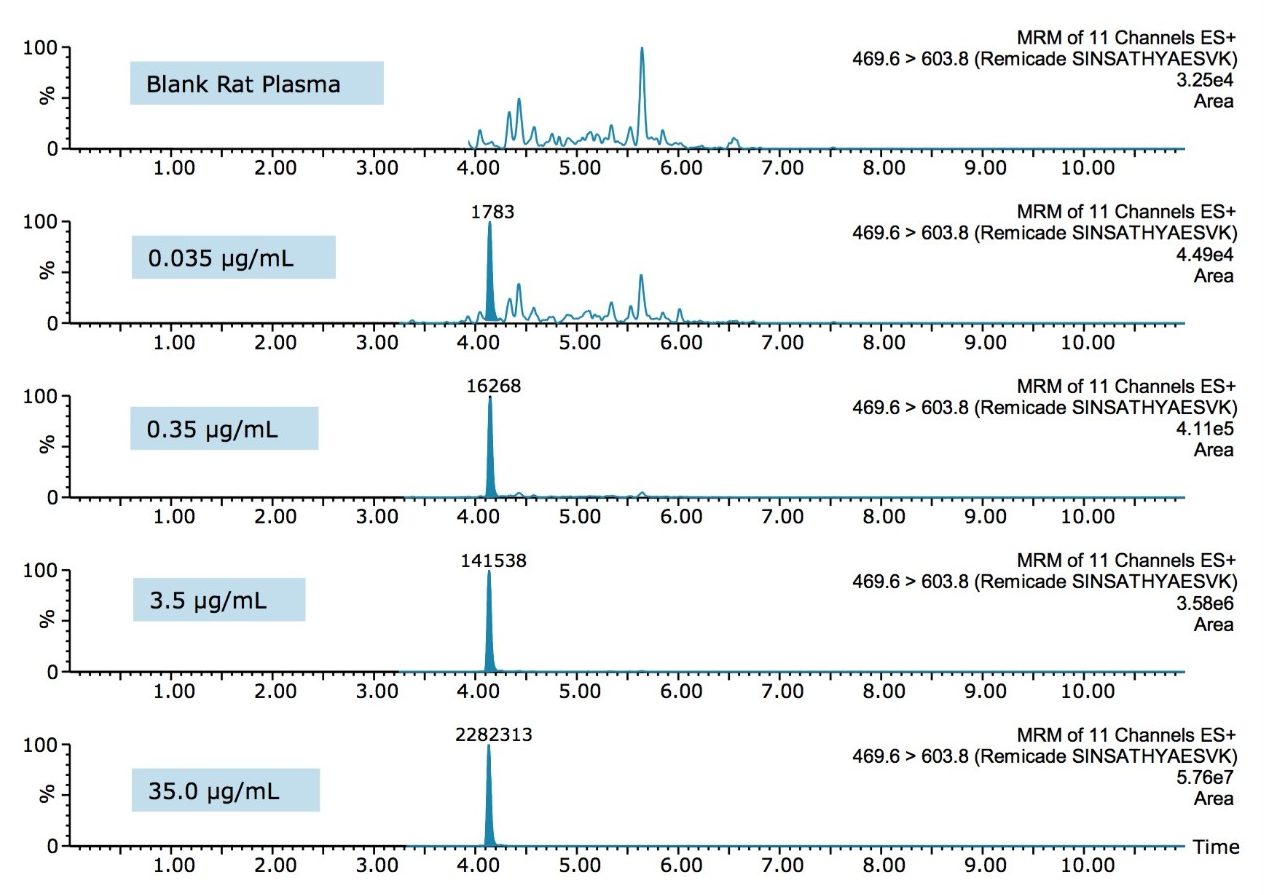

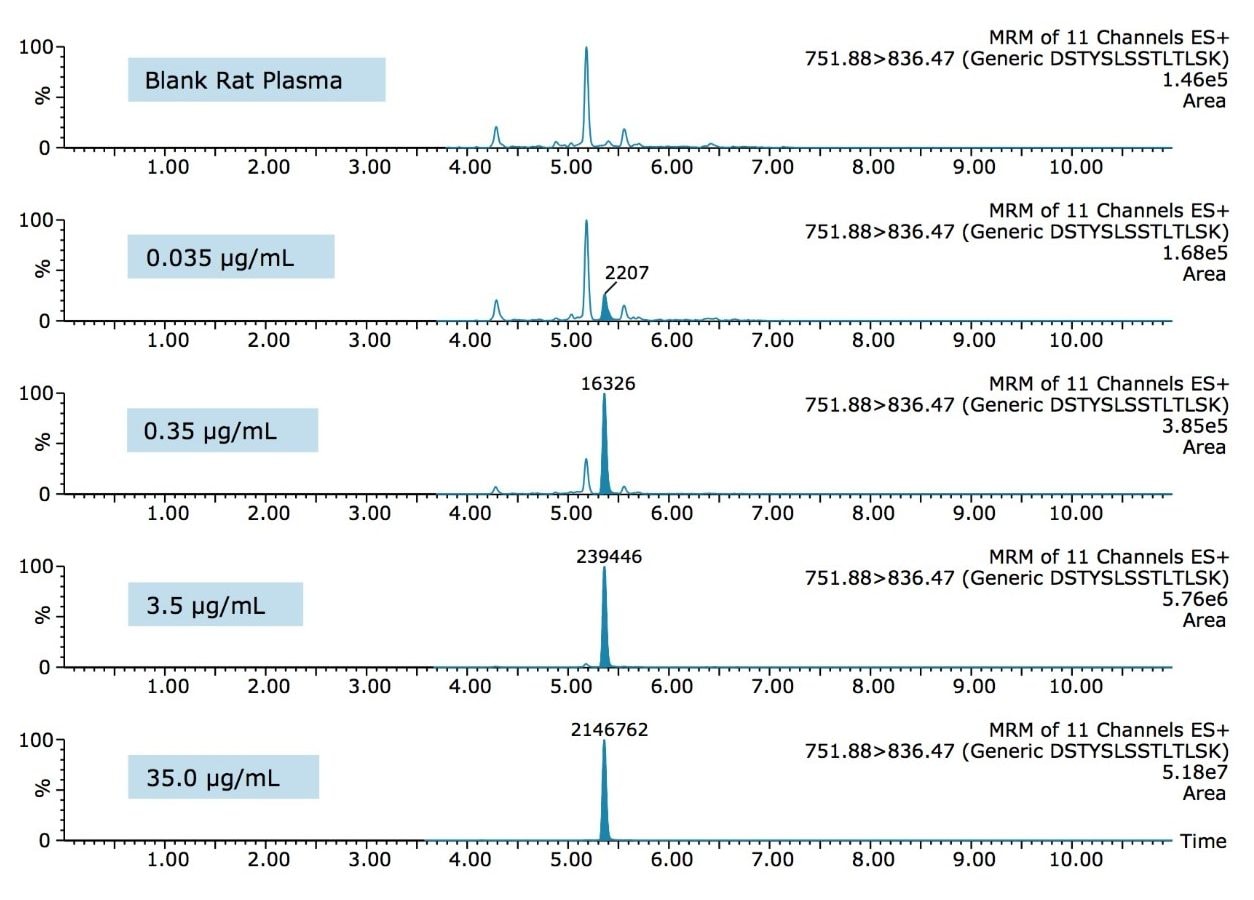
The ProteinWorks eXpress Digest Kit was successfully used to purify infliximab from a typical set of standard curve and QC samples in rat plasma. A limit of quantification of 10 ng/mL was readily achieved, while maintaining excellent linearity and single digit precision. The total sample prep time including an affinity purification step was under 6 hours. The total digest prep time was just over 2 hours. The universal, kit-based approach allows novice users to achieve ultra-low detection limits with a simple step-wise protocol and a set of standardized, pre-measured reagents, ensuring both the sensitivity required and the transferability desired of such methods.
720005535, November 2015