A Rapid and Sensitive Method for the Simultaneous Determination of Melamine and Cyanuric Acid in Infant Formulas, Adult Nutritional Products, and Protein Powders
Abstract
Benefits
- Simultaneous high-throughput screening of melamine (MEL) and cyanuric acid (CYA)
- Abundantly sensitive, extremely robust method – analyze more than 100 samples per day
- Method also applicable to the analysis of raw materials (e.g. casein or whey protein isolates) to verify the integrity of the protein supply prior to use in infant formula production
Introduction
Recent melamine contamination of milk, and milk containing products, has resulted in the illness of thousands of children in China, and the deaths of some. Melamine (1,3,5-triazine-2,4,6-triamine, CAS# 108–78–1) is commonly used in the production of plastics, adhesives, coatings, and flame retardants. Cyanuric acid (1,3,5-triazine-2,4,6-trione, CAS# 108–80–5), a structural analog of melamine, is commonly found as an impurity of melamine. The chemical structures of these compounds are shown in Figure 1.
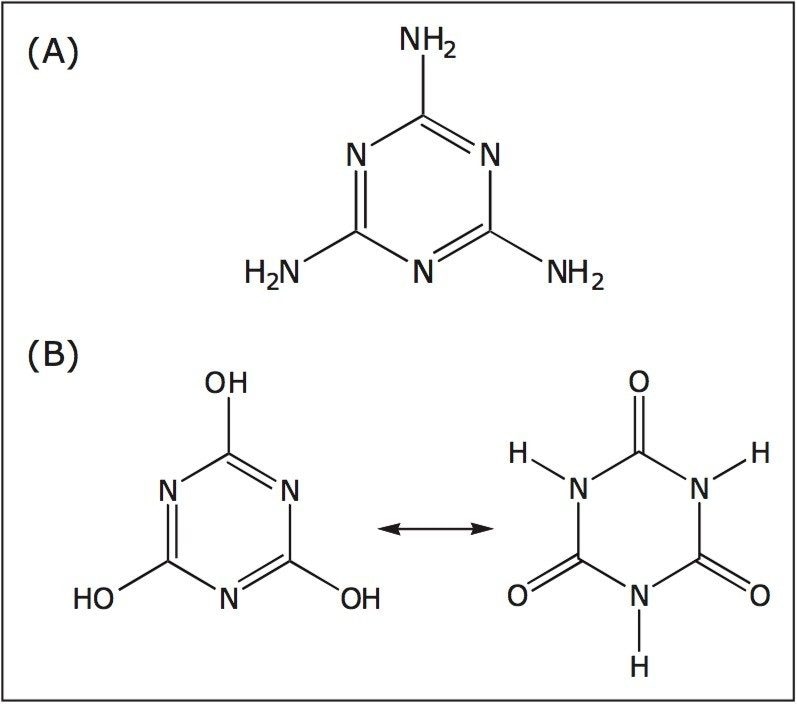
Given its high nitrogen content, melamine has been used to elevate the apparent protein content of milk in some areas of China. It is believed to be the combination of these two compounds that is responsible for renal toxicity. To date, many regulatory agencies have proposed limits of 1.0 ppm (µg/g) for infant formulas and 2.5 ppm (µg/g) for adult foods. The U.S. FDA has recently proposed that a tolerance of 1.0 ppm may be applied to the presence of either melamine or cyanuric acid in infant formula finished product, but states that they may not co-exist. Other agencies (e.g. Taiwan) have established limits for melamine as low as 0.05 ppm. Methods have been described for detection of melamine and cyanuric acid in flour1 and pet food2 and Shia and Diehl described the specific SPE extraction of melamine and cyanuric acid from infant formula.3
Experimental
UPLC Conditions
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH HILIC 2.1 x 150, 1.7 µm |
|
Mobile phase A: |
10 mM ammonium acetate in water |
|
Mobile phase B: |
10 mM ammonium acetate in 97/3 acetonitrile/water |
|
Weak needle wash: |
Methanol |
|
Strong needle wash: |
Water |
|
Needle type: |
PEEK |
|
Sample loop: |
10 µL |
|
Injection type: |
Full loop |
Gradient Table
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
Curve |
|
0.00 |
0.7 |
0.0 |
100 |
6 |
|
1.00 |
0.7 |
0.0 |
100 |
6 |
|
3.20 |
0.7 |
6.0 |
94.0 |
6 |
|
3.50 |
0.7 |
50.0 |
50.0 |
6 |
|
4.00 |
0.7 |
50.0 |
50.0 |
8 |
|
4.10 |
0.7 |
0.0 |
100 |
6 |
|
6.00 |
0.7 |
0.0 |
100 |
6 |
MS Conditions
|
MS system: |
ACQUITY® TQ Detector |
|
MS software: |
MassLynx |
See Table 1.
Melamine
|
Polarity: |
ESI+ |
|
Capillary voltage: |
3.50 kV |
Cyanuric Acid
|
Polarity: |
ESI- |
|
Capillary voltage: |
3.00 kV |
|
Source temperature: |
150 °C |
|
Desolvation temperature: |
400 °C |
|
Cone gas flow: |
50 L/hr |
|
Desolvation gas flow: |
900 L/Hr |
|
Collision gas flow: |
0.15 mL/min |
However, to assure the safety of infant formula, and to address regulatory testing requirements in the timeliest fashion possible, a new highly sensitive, more rapid test method has been developed for the simultaneous determination of melamine (MEL) and cyanuric acid (CYA) allowing high-throughput screening of product. The method is also applicable to the analysis of raw materials (e.g. casein or whey protein isolates) to verify the integrity of the protein supply prior to use in infant formula production. Isotopically labeled internal standards are used to normalize minor variability in sample preparation, as well as to correct for matrix effects in the instrumental response of the target compounds.
Independent stock solutions of melamine and cyanuric acid were prepared by dissolving in 100 mL of water at concentration of 100 µg/mL. A mixed intermediate standard was prepared by diluting the stock solutions in mobile phase B at concentrations of 2.5 µg/mL and 5.0 µg/mL for MEL and CYA respectively. Intermediate standards were prepared fresh daily.
sotopically labeled internal standards of melamine (13C3-15N3-melamine) and cyanuric acid (13C3-15N5-cyanuric acid) were purchased from Cambridge Isotope Laboratories as 0.1 mg/mL aqueous solutions. On the day of analysis, a mixed stable isotope standard solution was prepared by diluting the stable isotope internal standards in 2% formic acid in acetonitrile by combining the solutions in a 1:1:2 ratio.
Two transitions were monitored for each compound, including the stable isotope internal standards. The second transition was used as a confirmation, and the ratio of the peak area must match authentic standards to be considered a positive result.

Preparation protocol
- For raw materials, weigh 1.0 g of sample into a 20 mL glass scintillation vial
- Add 10.0 g water and mix well to dissolve sample – treat dissolved sample as a nutritional product sample
- Weigh 1.0 g product (powder or liquid) or prepared raw material into a 20 mL glass scintillation vial
- Add an aliquot of mixed internal standard solution
- Add 5 mL formic acid in water (2% v/v)
- Prepare sample by vortexing for approximately 15 seconds, or until all solids are dissolved
- In a second 20 mL glass scintillation vial, dispense 9 mL 97/3 acetonitrile/water containing 10 mM ammonium acetate
- Transfer 1 mL of the prepared sample into the 9 mL 97/3 acetonitrile/water solution
- Mix well
- Filter sample through a 0.2 µm PTFE syringe filter into an autosampler vial
Results and Discussion
Calibration curves for MEL and CYA were generated by plotting the response ratio of the target compound response/stable isotope internal standard response versus the concentration of the target compound. Linear calibration curves were generated for both MEL (0.25 to 40.0 ng/mL) and CYA (0.50 to 80.0 ng/mL) with correlation coefficients (R2) consistently greater than 0.99 on both instruments used. Calibration residuals for MEL and CYA were less than 10%. Typical calibration curves and residual plots are shown in Figure 2.

Method accuracy and precision were assessed using overspike samples of representative products and ingredients at concentrations above the estimated quantitation limit of the method. Finished products were spiked with 0.083 µg/g MEL and 0.568 µg/g CYA. Raw materials were spiked at 0.416 µg/g MEL and 1.26 µg/g CYA. Recoveries ranged from 90% to 110% for both MEL and CYA with <10% RSD for eight independent determinations acquired over four days on two instruments with multiple analysts. Results are summarized in Table 2.

Determination of the method detection limit (MDL) and method quantitation limit (MQL) was carried out according to the method described in 40 CFR Part 136 – Appendix B. The MDL is calculated from the standard deviation of the determined concentrations and the MQL is defined as three times the MDL. Following the analysis of separate overspikes on two instruments over a period of eight days, the method quantitation limits for MEL and CYA were established as 0.039 µg/g and 0.105 µg/g, respectively. Due to the dilution required for the analysis of raw materials, detection limits are approximately 10-fold higher.
Tandem mass spectrometry allows selective detection in selected reaction monitoring (or multiple reaction monitoring) mode. In this mode of operation, only compounds that possess the appropriate precursor/product ion pair were detected. As seen in Figure 3, the product ion spectrum for MEL contains two primary ions.

The specificity of the method was increased by monitoring both MS/MS transitions in the analysis of MEL. Positive identification of MEL is made if a peak is present in both the 127→85 and 127→68 MRM chromatograms, and the peak response ratio must be within 20% of that observed in standards. Representative chromatograms for standards (0.25 ng/mL MEL and 2.0 ng/mL CYA), an unfortified infant formula and a fortified infant formula (0.083 µg/g MEL and 0.568 µg/g), are shown in Figure 4.

The use of stable isotope internal standards greatly enhances the quantitative performance and robustness of the method. Using this simple dilute and shoot procedure, over 100 samples per day can be tested for the presence of MEL and CYA using tandem quad detection. The method proved to be suitable for the analysis of over 95% of the infant formulas, nutritional products, and protein powders tested. In other cases, or for complex matrices where co-extracting interferences are present, an additional solid-phase extraction (SPE) step, involving either cation or anion exchange SPE, should be employed.
Conclusion
A rapid and robust method for the simultaneous determination of melamine and cyanuric acid in liquid and powder infant formula and adult nutritional products has been developed and validated according 40 CFR Part 136. Detection and quantitation limits were 0.013 µg/g and 0.039 µg/g for melamine, and were 0.035 µg/g and 0.105 µg/g for cyanuric acid in finished products. Average recovery and precision of overspikes (n=48, eight days, two instruments) was 100.9%, with 6.8% RSD for melamine (0.083 µg/g), and 99.1%, with 10.4% RSD for cyanuric acid 0.568 µg/g).
Following a simple dilution, the ACQUITY UPLC System combined with the TQ Detector provided a rapid (less than 6 minute) analysis for both melamine and cyanuric acid. The combination of UPLC® with tandem MS detection provides both excellent sensitivity and a throughput greater than 100 samples per day. The addition of stable isotope internal standards provides good linearity and quantitation across a wide concentration range and a variety of product types, resulting in confident identification and quantitation of melamine and cyanuric acid.
The method described provides the ability to rapidly screen protein ingredients, milk supplies, and nutritional products, including infant formula, to enable both producers and regulatory bodies to assure the safety and integrity of the products.
Addendum
While this application was developed on Waters instrumentation, it was not developed by Waters scientists. Rather, it was developed by scientists at a major infant formula manufacturer and is currently being employed by them to ensure the safety of their products, surpassing the regulatory requirements with the highest throughput achievable. The method has application for the detection of melamine and its analogue cyanuric acid in infant formula and the ingredients used during its production.
We at Waters believe that this is an important body of work of particular relevance to manufacturers involved in the production of infant formula and to regulators alike. We are grateful to our customer for agreeing to allow us to share the details of the method.
We realize that the demands and requirements for detecting melamine and its analogues continue to develop rapidly, and there exists the need for methods with the ability to detect these substances in an ever-increasing range of matrices of varying complexity. This is particularly true in light of the recent USDA notification informing of their intent to collect at the retail level and analyze a range of meat and poultry products containing milk-derived ingredients. Waters scientists continue to address this through development of procedures employing specific solid phase extraction for those matrices of challenging complexity. Details of these existing, alternative extraction procedures can be found on the Waters website, as can details of new methods as they become available.
References
- S Ehling, S Tefera, I P Ho. Food Additive and Contaminants. Dec 2007; 24(12): 1319–1325.
- M Benvenuti, A O’Connor. Melamine, Ammeline, and Cyanuric Acid Analysis by UPLC/MS/MS and UPLC/PDA. Waters Application Note. 720002300, 2007.
- J Shia, D Diehl. Protecting the Food Supply: Rapid, Specific Analysis of Melamine and Cyanuric Acid in Infant Formula by LC/MS/MS, Waters Application Note. 720002865, 2008.
720002889, May 2015