
The application of gas chromatography coupled to mass spectrometry (GC-MS) is well established and documented for the analysis of ubiquitous environmental contaminants, such as persistent organic pollutants (POPs). Four classes of globally regulated POPs are polycyclic aromatic hydrocarbons (PAHs),polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides (OCPs).
Due to the structural similarities of many POP congeners and the complexity of these compounds, their analysis can prove challenging. Traditional GC-MS and MS/MS techniques by electron impact (EI) ionization have been favored, due to the volatility, thermal stability, and non-polarity of the compound.
However, given the hard ionization associated with EI, extensive fragmentation can impact the abundance of the molecular ion and compound specific spectra. Atmospheric pressure gas chromatography (APGC) allows for a softer ionization technique, thus providing a more abundant molecular ion.
In this application note, we describe the development and validation of a quantitative method for 141 multi-class POP compounds in a variety of foodstuffs to ensure continued monitoring and consumer safety in Quebec, Canada.
The sensitivity, selectivity, and quantification capability of APGC coupled with Waters Xevo TQ-S is described, along with a generic sample preparation method for the extraction of all analytes from a variety of food matrices.
The application of gas chromatography coupled to mass spectrometry (GC-MS) is well established and documented for the analysis of ubiquitous environmental contaminants, such as persistent organic pollutants (POPs). Four classes of globally regulated POPs are polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides (OCPs).
Common physicochemical characteristics of these POPs include resistance to chemical and biological degradation and high lipophilicity, thus resulting in their persistence and significant potential to bioaccumulate. Many of these compounds are classified as toxic, carcinogenic, and probable carcinogenic. Such is the concern regarding these pollutants that international treaties, in association with regional legislation, requires continued monitoring to ensure human safety.
Due to the structural similarities of many POP congeners and the complexity of these compounds, their analysis can prove challenging. Traditional GC-MS and MS/MS techniques by electron impact (EI) ionization have been favored, due to the volatility, thermal stability, and non-polarity of the compound. However, given the hard ionization associated with EI, extensive fragmentation can impact the abundance of the molecular ion and compound specific spectra. Atmospheric pressure gas chromatography (APGC) allows for a softer ionization technique, thus providing a more abundant molecular ion. Operated at atmospheric pressure, compounds are ionized by corona discharge in the presence of nitrogen. This ionization reaction, depending on the analytes of interest, can occur by two processes: charge transfer (under dry conditions) and proton transfer (in the presence of a protic solvent).
In this application note, we describe the development and validation of a quantitative method for 141 multi-class POP compounds in a variety of foodstuffs to ensure continued monitoring and consumer safety in Quebec, Canada. The sensitivity, selectivity, and quantification capability of APGC, when coupled with the Waters Xevo TQ-S will be determined, using a generic sample preparation method for the satisfactory extraction of all analytes from a variety of food matrices.
|
GC system: |
7890A |
||
|
Injector: |
Splitless |
||
|
Injection: |
1 μL |
||
|
Temp.: |
300 °C |
||
|
Column: |
DB-5 (J&W, USA) 30 m x I.D. 0.25 mm x df 0.25 μm |
||
|
Guard column: |
120 cm fused silica hi-temp |
||
|
Interface: |
55 cm fused silica hi-temp |
||
|
Temp. gradient: |
70 °C (hold for 1 min), 12 °C.min-1 to 250 °C, 5 °C.min-1 to 280 °C, 4 °C.min-1 to 310 °C (hold for 4.5 min) |
||
|
Transfer line temp.: |
340 °C |
||
|
Carrier gas flow: |
1.5 mL.min-1 (helium) |
||
|
Auxiliary gas: |
350 l.h-1 (nitrogen) |
||
|
Make-up gas: |
250 mL.min-1 (nitrogen) |
|
MS system: |
Xevo TQ-S |
||
|
APCI corona pin current: |
2.5 μA |
||
|
Cone gas: |
250 l.h-1 (nitrogen) |
||
|
Acquisition mode: |
multiple reaction monitoring (MRM) in positive ionization for all four classes of POPs shown in Table 3 |
||
|
Data management: |
MassLynx Software with TargetLynx Application Manager |
Milk, infant formula, beef, pork, chicken, and fish were analyzed for PCB, PBDE, OCP, and PAH compounds using the following generic extraction procedure. Homogenized sample (12 g) was placed in a 50-mL glass centrifuge tube and fortified with internal standard, as described in Table 1. A smaller sample portion (10 g) was weighed for foods with high fat content (e.g. beef). Water (5 mL) was added to the solid food samples and reconstituted powder milk formulae. Samples were vortexed and allowed to stand for 20 minutes. Ethyl acetate (10 mL) was added and samples were shaken vigorously for 1 minute. QuEChERS salts, magnesium sulphate (4 g), and sodium chloride (2 g) were added to the tubes and shaken vigorously for an additional minute.
Following centrifugation, the supernatant (5 mL) was removed, evaporated, and reconstituted in dichloromethane (2.4 mL). It was then filtered through 0.45 µm PTFE filters in preparation for gel permeation chromatography (GPC). EnvirosepABC GPC pre column (60 x 21.2 mm) and column (350 x 21.2 mm) were used, with dichloromethane as eluent (5 mL.min-1). The resultant extract was transferred to a suitable tube for evaporation, where the GPC collection tube was rinsed three times with dichloromethane. These rinses were combined with the original extract and evaporated to 750 µL. The volume was then made up to 1 mL in hexane and silica gel cleanup was performed. Silica columns were prepared by adding silica (2 g) into a 1-cm wide borosilicate glass column with a glass wool frit. These columns were conditioned with 3:1 hexane: dichloromethane solution (12 mL), followed by hexane (8 mL). The samples were loaded and eluted using 3:1 hexane: dichloromethane solution (20 mL). These extracts were evaporated to <0.5 mL, and fortified once more with the internal standard (25 µL, compounds marked with ** in Table 1). All samples were made up to 500 µL volume with isooctane, vortexed, and analyzed using the Xevo TQ-S with APGC.
Method efficiency was determined and validated based on an in-house document that was inspired by several internationally recognized documents.5-15 The limits of detection (LODs) and limits of quantification (LOQs) were determined using fortified replicates (n= 10) for all analytes in each matrix. This was carried out in accordance with the IUPAC method, i.e. LOD= 3 x std deviation of experimental noise, and LOQ= 10 x standard deviation of experimental noise.
The lowest limit (LL) was determined for all analytes where subsequent validation work and statistical analysis were based on this. Analyte recovery, repeatability (%RSD), and linearity were investigated. Replicate samples (n= 9) were prepared for each matrix (n= 6) at three fortification levels: 0.2 x LL, LL, and 2 x LL. From these replicates, the recovery and method repeatability were determined individually for each matrix.
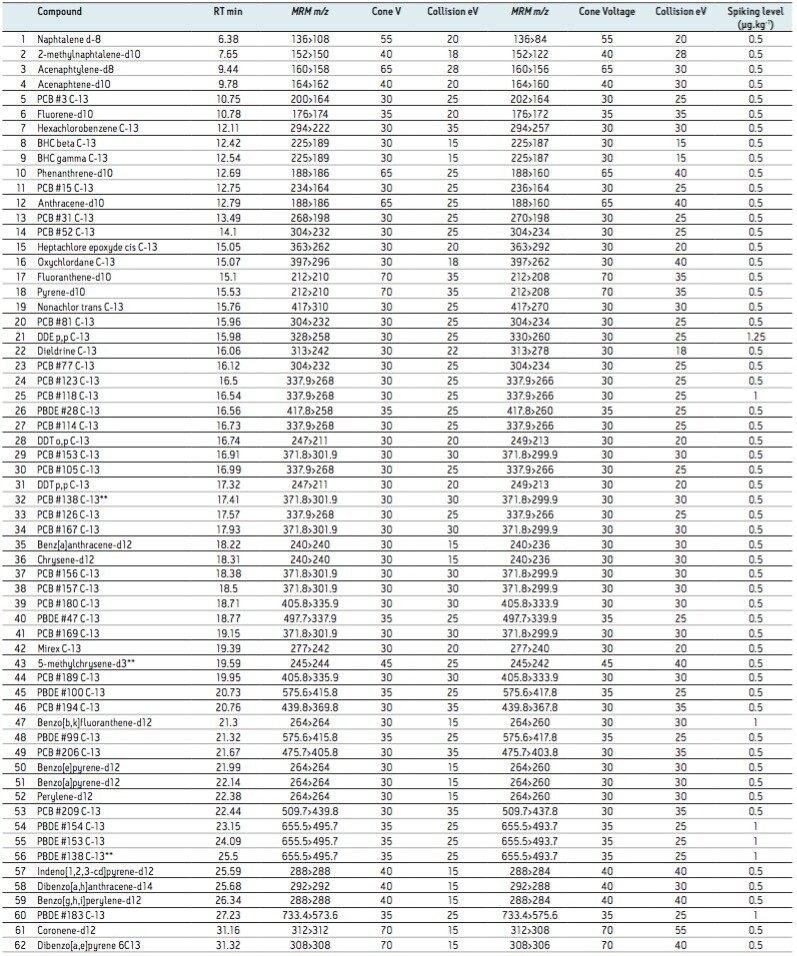
Table 1. Internal standards for each class of POP, in order of retention time.
**Signifies internal standards added prior to injection.
The Xevo TQ-S with APGC was evaluated as an accurate and sensitive instrument for the detection of multi-class POP compounds (PAHs, PCBs, PBDEs, and OCPs). Over 140 analytes (excluding internal standards) were targeted in this method and represent the most common congener mixes and regulated POP compounds, covering low, medium, and high boiling compounds.
The accurate detection and quantification of certain compounds, including coronene, dibenzo pyrenes, and BDE #183 can often prove challenging during traditional GC-EI-MS analysis. Despite their complex structures and higher boiling points, these compounds are readily analyzed by APGC, where excellent LODs were achieved, as shown in Table 3.
A generic extraction method was developed for all analytes using the internal standards described in Table 1. Excellent recoveries, linearity, and LODs were determined for all analytes across the six different matrices. Comparable recoveries, with satisfactory repeatability, were achieved for the analytes in all matrices, as shown in the Table 2, using the recovery of PCB #126 as a representative example.
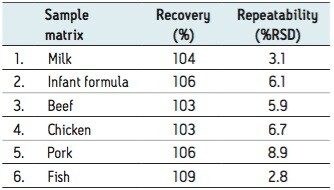
The validated method using TQ-S with APGC was submitted and successfully accredited in accordance with international standard ISO 17025. For ease of discussion, the method’s results for multi-class analytes will be demonstrated from here on using pork meat. The results shown in Table 3 focus on analyte recoveries and repeatability at the lowest fortification level in order to demonstrate system sensitivity and robustness at trace levels. Furthermore, the linearity and limits of detection, as summarized in Table 3 are discussed.
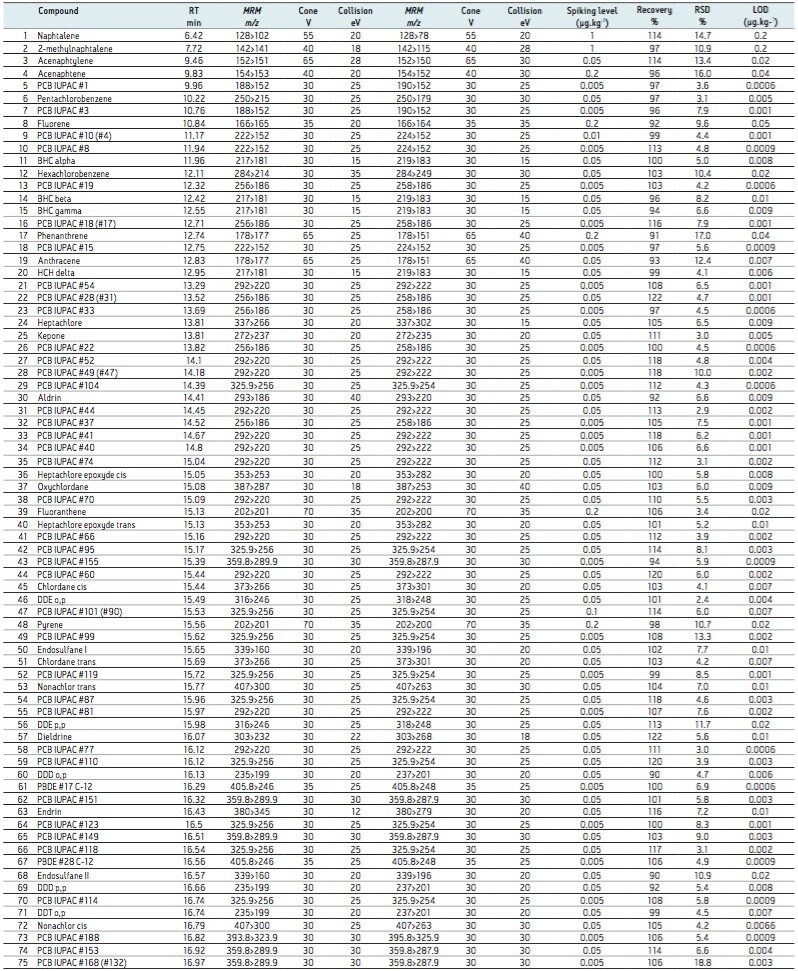
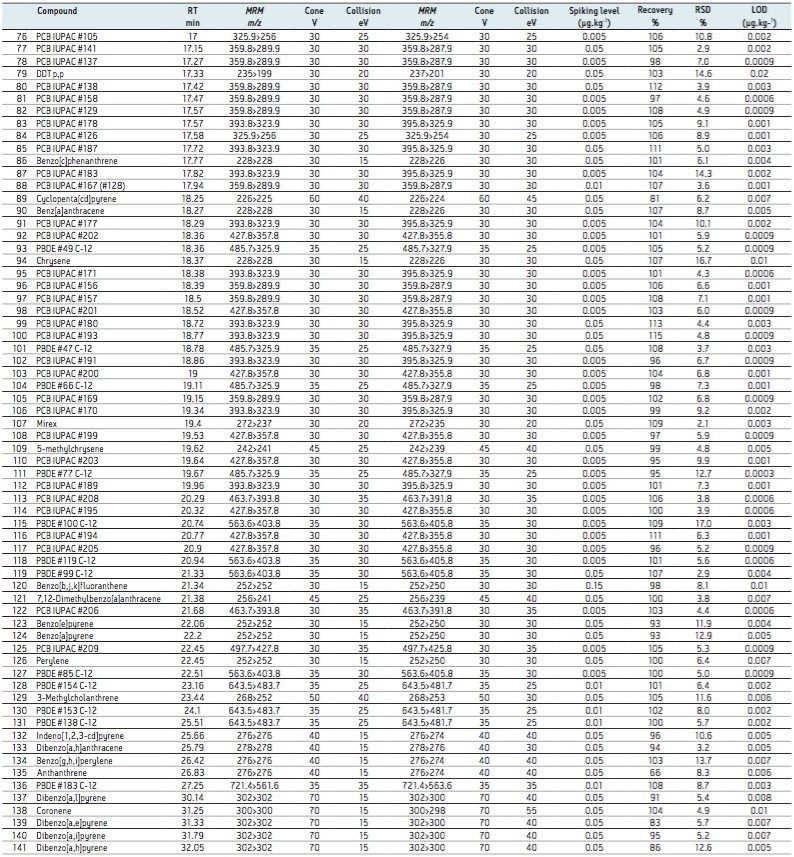
Given the softer ionization associated with APGC, more abundant molecular ions can be observed compared with traditional EI spectra. During method development stages, all of the analytes showed better sensitivity for the molecular ion by charge transfer (in dry source) in comparison with protonation, given the electronegativity associated with chlorinated and brominated compounds.
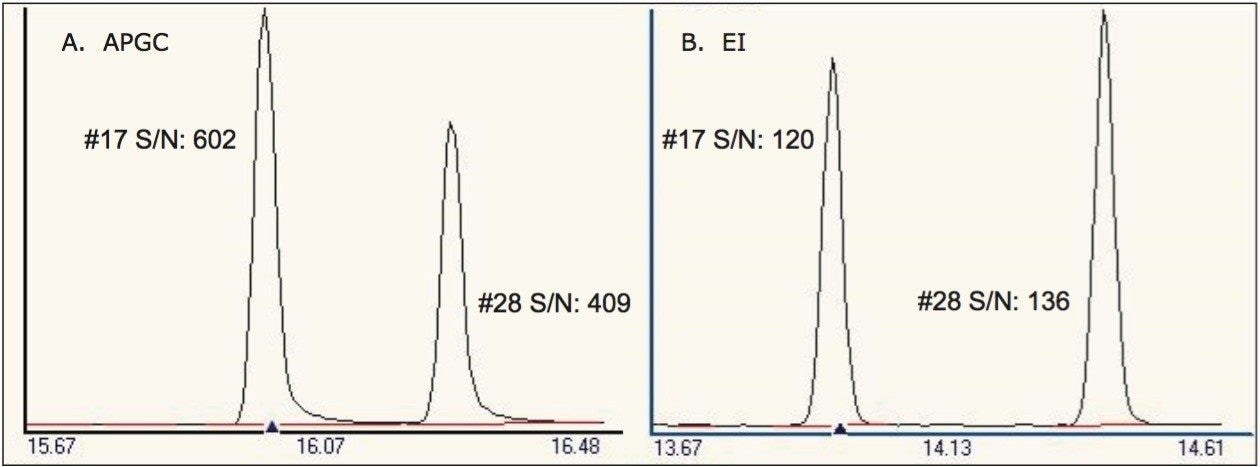
The increased sensitivity observed for many POP analytes, in comparison with traditional GC-EI-MS methods can be seen in Figure 1. Here the signal-to-noise ratio (S/N) determined for BDE #17 and #28 shows a significant increase when analyzed by APGC. This further reduces matrix loading, thus improving liner, column life, and instrument robustness, while reducing instrument maintenance.
The medium and high concentration replicates were combined (n= 18) to allow for an averaged and more representative statistical analysis of the method recovery and repeatability for each matrix. Further statistical analysis was completed to allow for validation of a robust method at low concentration levels. To this end, the method recovery and repeatability were determined separately for all analytes in each matrix at the lowest level of fortification (0.2 x LL, where n= 9). These results are shown for pork matrix in Table 3.
The detection of POPs in food and environmental samples are challenging, because of their ubiquitous presence and the increasingly low detection levels required to meet regulatory limits in complex matrices. However, the analysis of PBDEs, PCBs, PAHs, and OCPs can be achieved below all the required levels of detection using APGC-MS.
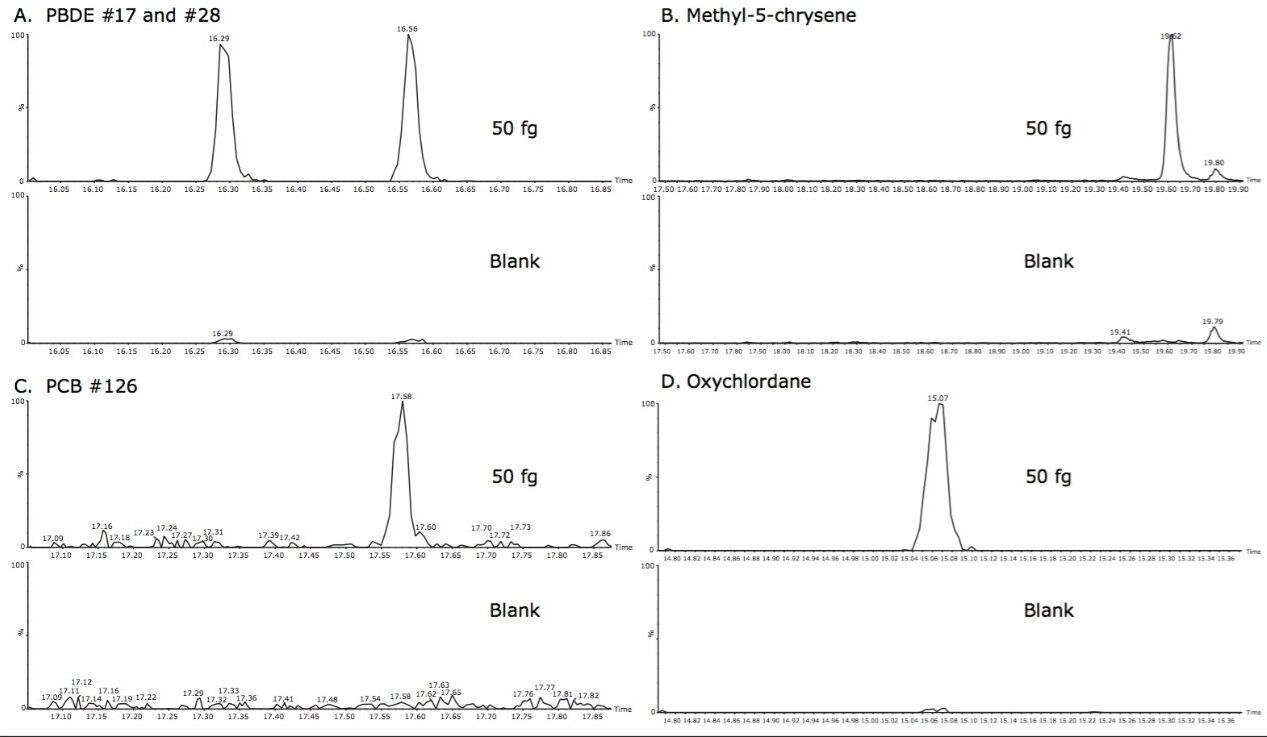
The limits determined for all of the analytes are summarized in Table 3, where concentrations of <1 µg.kg-1 were achieved for all analytes in pork extract. The excellent sensitivity achieved is further demonstrated in Figure 2, where the S/N ratio was determined for 50 fg on column. An example of each class of POP analyzed was compared to its matrix blank, thus demonstrating the selectivity afforded.
The developed method allowed for excellent repeatability for the multi-class components fortified at low levels in a variety of matrices. This is well demonstrated by the validation data shown in Table 3, where excellent recoveries and method repeatability are shown for all analytes fortified in pork meat (n= 9) at levels between 50 to 1000 ng.kg-1.
Using the optimized generic sample preparation and cleanup method, the percentage recoveries ranged from 65% to 122% in pork matrix. Percentage relative standard deviations (%RSD) were found to be <20% for all analytes. This is an acceptable level for multi-residue analysis in complex matrices, showing low variance for all of the PBDEs, PCBs, PAHs, and OCPs when spiked at parts per trillion (ppt, equating to ng.kg-1) levels in the complex matrix.
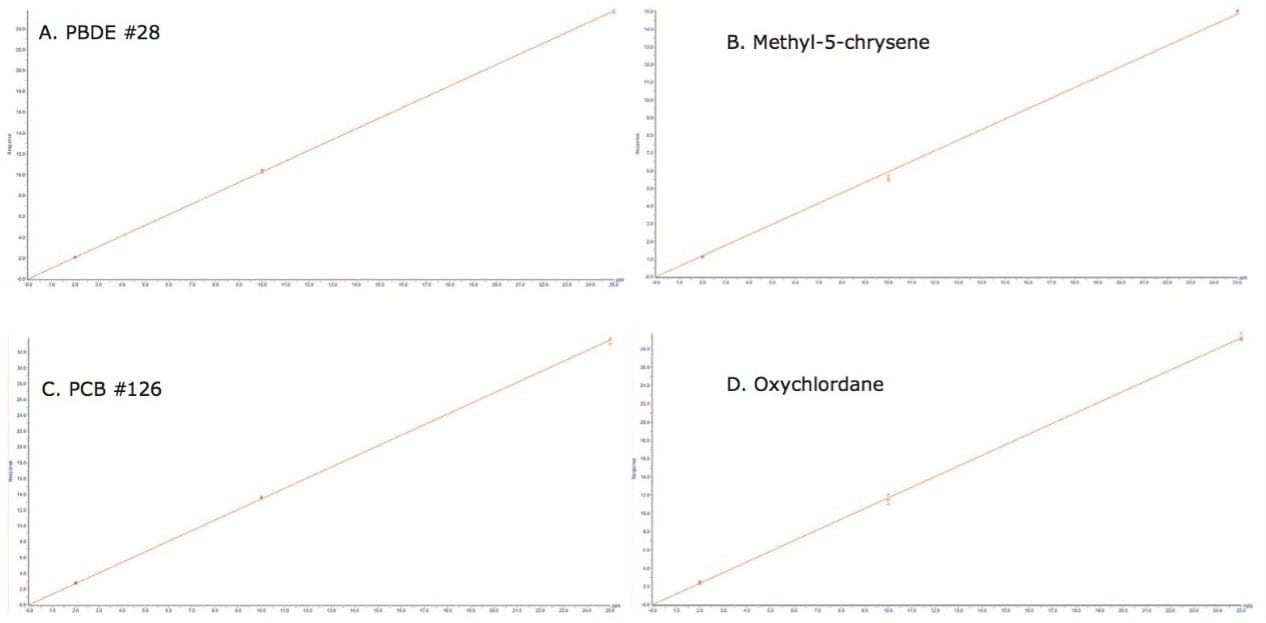
Linearity was investigated for all analytes utilizing the internal standards described in Table 1. Good correlation was achieved (R2>0.99) over a satisfactory working range of 2 to 25 µg.kg-1. This working range was deemed most appropriate, allowing for the accurate quantification for all analytes at legislated levels where applicable. An example of the calibration curves achieved utilizing the internal standard is provided in Figure 3 for each class of POP in pork meat.
Time-consuming and costly analyses are a major drain on food and environmental testing laboratories, where multi-analyte methods are preferred for efficient use of resources. While the robust Xevo TQ-S can be coupled with UPLC to provide sensitive analysis of LC-amenable compounds, this work shows the ability of the Xevo TQ-S to analyze multi class POPs by atmospheric pressure gas chromatography with excellent robust sensitivity.
The optimization of a single cleanup method for a variety of analytes has been shown to achieve satisfactory recoveries, while allowing the Xevo TQ-S with APGC to quantify analytes below the regulatory limit. Taking pork as an example, excellent recoveries, in the range of 66% to 122% were determined, where the repeatability was <20% for all 141 POP analytes.
This validated and accredited method has been implemented by the MAPAQ for the routine analysis of a multitude of meats, fish, milk, and infant formula to ensure consumer safety in Quebec, Canada. When compared with traditional GC-EI-MS methods, increased sensitivity, less maintenance, and routine cleaning has been required for the Xevo TQ-S with APGC, further improving laboratory efficiency.
MAPAQ wishes to acknowledge the skills and day-to-day work of laboratory technicians Justine Legros and Anne-Marie Simard, along with the help of three one year internship students: Pierre-Luc Cloutier (2011), Renaud Lussier (2012), and Gabrielle Mercier (2013).
720005144, August 2014