The USP compendial method for levonorgestrel and ethinyl estradiol tablets was successfully transferred from HPLC to UPLC using scalable column chemistries and the ACQUITY UPLC Columns Calculator. The UPLC method is approximately 85% faster than the HPLC method and results in a 92% savings in sample amount injected and mobile-phase solvent consumption. While extended centrifugation of the tablet sample was helpful in preparing a better quality sample for injection, it did not fully alleviate the increased pressure seen in both HPLC and UPLC during a routine use study. Instead, incorporation of a gradient wash to the isocratic method aided in preventing sample build-up on column, thereby stabilizing the pressure during routine use evaluations. Routine use of the UPLC transferred USP method was evaluated using formulated tablet samples on an ACQUITY UPLC BEH C8, 1.7 μm column. After 2200 injections, the column still passed all USP assay suitability specifications for levonorgestrel and ethinyl estradiol tablets, demonstrating that extended column performance is achievable for high throughput analysis of generic tablet formulations using an isocratic USP monograph method.
Chromatographic assays for the analysis of generic drugs are frequently based on USP compendial methods. The HPLC methods described in the USP are not routinely updated and do not take advantage of sub-2 μm particle technology, which provides faster run times and increased productivity of analysis. Additionally, routine analysis of drug formulations can result in more frequent and costly replacement of columns due to the chemical diversity of excipients and other formulation agents. USP methods developed on HPLC are often isocratic to eliminate the need for re-equilibration, thereby increasing sample throughput. However, if the sample is not properly eluted off of the column, it can build up on the column bed, resulting in increased backpressure and potentially premature failure of the column.
This application note will first compare the USP method for levonorgestrel and ethinyl estradiol tablets using two different L7 HPLC columns. The method is then transferred to UPLC using a UPLC column with the same stationary phase. The use of UPLC and sub-2μm particle columns allows for a significantly faster analysis while still meeting the system suitability criteria specified in the USP monograph. Finally, suggestions are offered to alleviate the increased backpressure that may be observed when repeatedly analyzing formulated levonorgestrel-ethinyl estradiol tablet samples in a typical quality control laboratory.
|
Mobile Phase: |
7:3:9 acetonitrile:methanol:water |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 215 nm |
|
USP Column: |
Zorbax C8, 4.6 x 150 mm, 5 μm (USP designation: L7); XBridge C8, 4.6 x 150 mm, 5 μm (USP designation: L7), part number 186003017 |
|
Needle Wash: |
acetonitrile |
|
Sample Purge: |
acetonitrile |
|
Seal Wash: |
50:50 methanol:water |
|
Flow Rate: |
1 mL/min |
|
Injection Volume: |
50 μL |
|
Mobile Phase: |
7:3:9 acetonitrile:methanol:water |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 215 nm |
|
USP Column: |
ACQUITY UPLC BEH C8, 2.1 x 50 mm, 1.7 μm (USP designation: L7), part number 186002877 |
|
Needle Wash: |
acetonitrile |
|
Sample Purge: |
acetonitrile |
|
Seal Wash: |
50:50 methanol:water |
|
Flow Rate: |
0.61 mL/min |
|
Injection Volume: |
3.5 μL |
|
Data Management: |
Empower 2 CDS |
|
USP Resolution: |
NLT 2.5 |
|
Peak Area RSD: |
NMT 2.0% |
Sample Preparation
Standard:
Levonorgestrel, 15 μg/mL and ethinyl estradiol, 3 μg/mL in mobile phase (Waters Analytical Standard).
Sample:
Dissolve levonorgestrel and ethinyl estradiol commercially-available tablets in mobile phase to a final concentration of 15 μg/mL levonorgestrel and 3 μg/mL ethinyl estradiol. Sonicate for 5 minutes, shake mechanically for 20 minutes. Centrifuge at 4000 rpm for 10 minutes. Collect supernatant and re-centrifuge at 12,000 rpm for 30 minutes, pipet clear supernatant for injection.
Samples were prepared according to the USP compendial assay method for levonorgestrel and ethinyl estradiol tablets.1 Samples were first centrifuged at 4000 rpm for 10 minutes and yielded a pale cloudy solution. Next, an aliquot of sample was filtered through a 0.2 μm PTFE filter, but the filtrate remained cloudy due to the extremely fine nature of the particulates in the sample and further filtration was not pursued. Samples were instead centrifuged at 12,000 rpm for 30 minutes and the supernatant was collected, yielding a clear solution for injection.
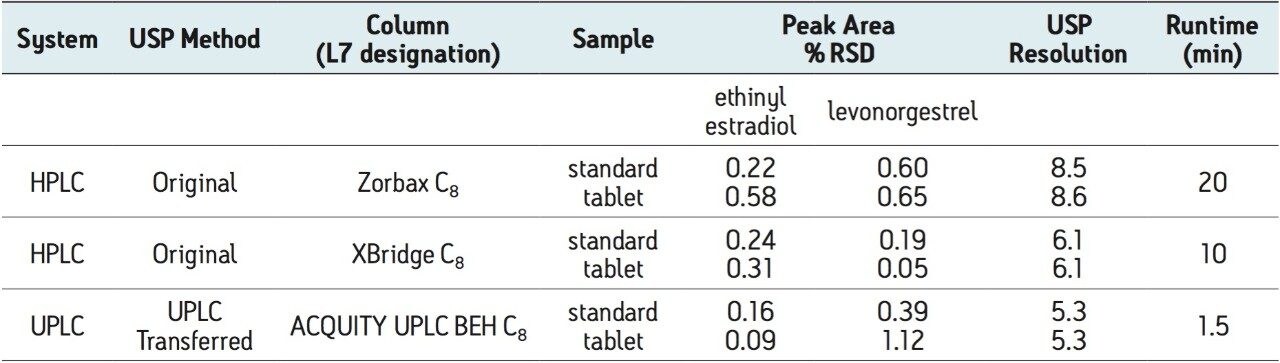
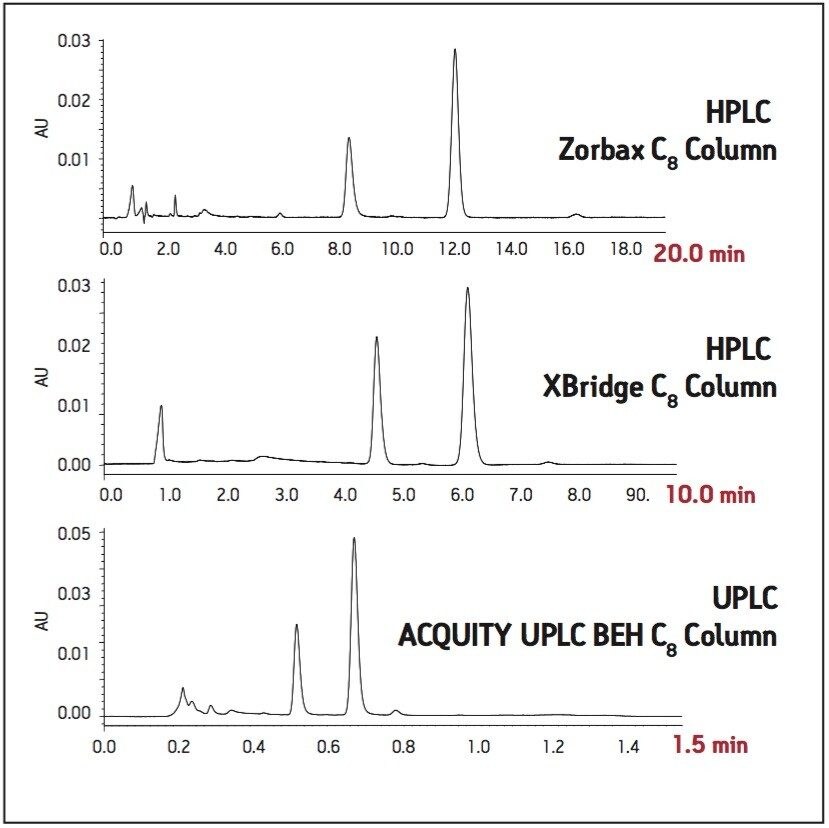
The USP method for levonorgestrel and ethinyl estradiol tablets requires the use of an L7 column and suggests an Agilent Zorbax C8 column. This column was tested per the USP assay method on an Alliance HPLC system, with five replicate injections of both levonorgestrel-ethinyl estradiol standard and tablets. The samples were also run on the HPLC system using a Waters XBridge C8 column. This column was chosen since it has similar selectivity to the Zorbax C8 column and it has an equivalent UPLC column chemistry (ACQUITY UPLC BEH C8), facilitating direct method transfer to UPLC. Chromatograms comparing the USP method using the Zorbax and XBridge C8 HPLC columns are compared in Figure 1. The columns show similar selectivity, but the Zorbax C8 column shows greater overall retention of the two active ingredients compared to the XBridge C8 column. However, the faster elution of analytes using the XBridge column allows for a much shorter analysis time, while sacrificing only a small amount of resolution. All of the USP assay suitability results were well within the specified criteria limits for both columns (Table 1). The effect of retentivity on productivity in the high throughput analysis of generic drugs is important to consider, even within the same USP column designation categories.
Assay Suitability Criteria
USP Resolution (between 2 peaks): NLT 2.5
Peak Area RSD: NMT 2.0%
Table 1. Assay suitability results comparing HPLC to UPLC for five replicate injections of levonorgestrel and ethinyl estradiol standard and tablet samples
Next, the USP assay method was transferred from HPLC to UPLC using the ACQUITY UPLC Columns Calculator.2 Scaling calculations were performed accounting for particle size and the method was scaled from the XBridge C8 HPLC column to an ACQUITY UPLC BEH C8, 1.7 μm column. Both columns have the same stationary phase chemistry and only differ in particle size. Five replicate injections of both levonorgestrel and ethinyl estradiol tablets and standard were analyzed separately. Assay suitability criteria including %RSD for peak area, and USP resolution between ethinyl estradiol and levonorgestrel peaks were compared between HPLC and UPLC. A comparison of both systems is shown in Table 1, where the UPLC transferred method clearly passes all system suitability criteria. The run time of the UPLC method is 1.5 minutes compared to a 10-minute HPLC method, resulting in an approximate 85% savings in analysis time and 92% savings in solvent consumption and sample injected (Figure 1).
In order to evaluate the performance of the UPLC transferred USP method with high-throughput analysis of formulated tablet samples, a routine use study was performed using the ACQUITY UPLC BEH C8, 1.7 μm column.
Levonorgestrel and ethinyl estradiol tablet samples were injected along with a standard mixture of levonorgestrel and ethinyl estradiol as a bracketing standard to simulate routine use in a typical quality control (QC) laboratory. Five replicate injections of standard were followed by twenty replicate injections of formulated tablet sample and this cycle of injections was repeated continuously until assay suitability criteria no longer passed. Pressure, peak area, retention time and USP resolution between the two peaks (levonorgestrel and ethinyl estradiol) were monitored throughout the study.
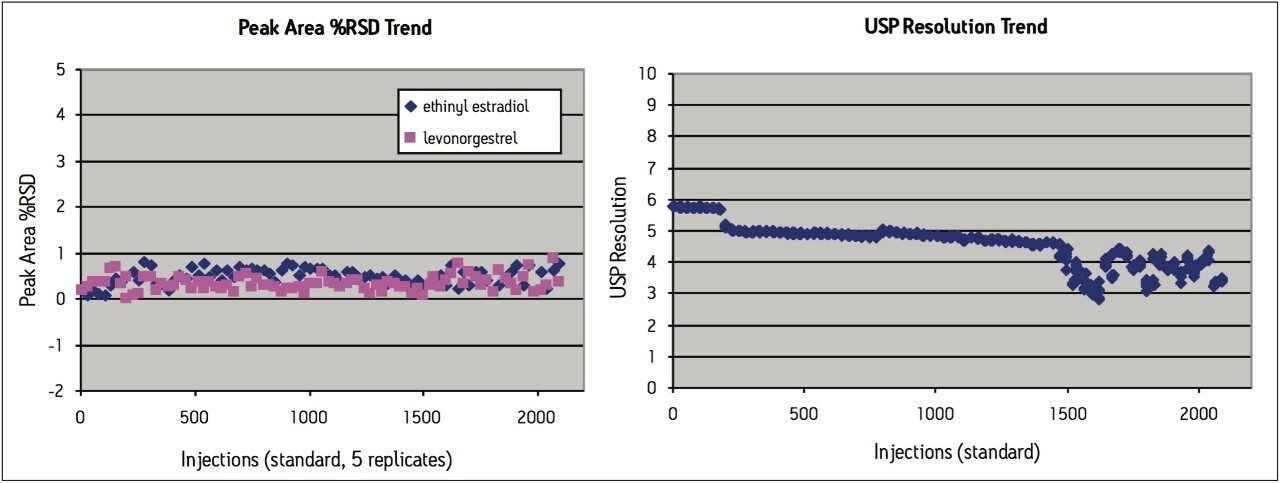
Peak area RSD remained within 2.0% for levonorgestrel and ethinyl estradiol standard injections, and USP resolution remained greater than 2.5 throughout the study, which is within assay specifications (Figure 2). Backpressure increased steadily throughout the study (Figure 3a), and increased approximately 36% from 7200 psi to 9800 psi over the first 1000 injections. The overall pressure trend for the UPLC routine use study was compared to a routine use evaluation for the original USP method run on an Alliance HPLC system using the Zorbax C8 column. On the HPLC system (Figure 3b), the pressure began near 1350 psi and rose to approximately 2350 psi over 1000 injections, a 74% increase. Both the original USP method on HPLC, and the UPLC transferred method for levonorgestrel and ethinyl estradiol exhibited a gradual increase in pressure using different columns and different systems. For this reason, the pressure increase is attributed to the sample, likely due to poor aqueous solubility of the hydrophobic steroids and sample formulation components in the aqueous mobile phase, which contains only about 50% organic solvent. While the pressure increase on the ACQUITY UPLC system is noticeable, the relative pressure increase is far below that of the HPLC system using the Zorbax column. Despite the observed pressure increases throughout the study, both systems were well within the running pressure limits of the instrumentation and all system suitability criteria were met.
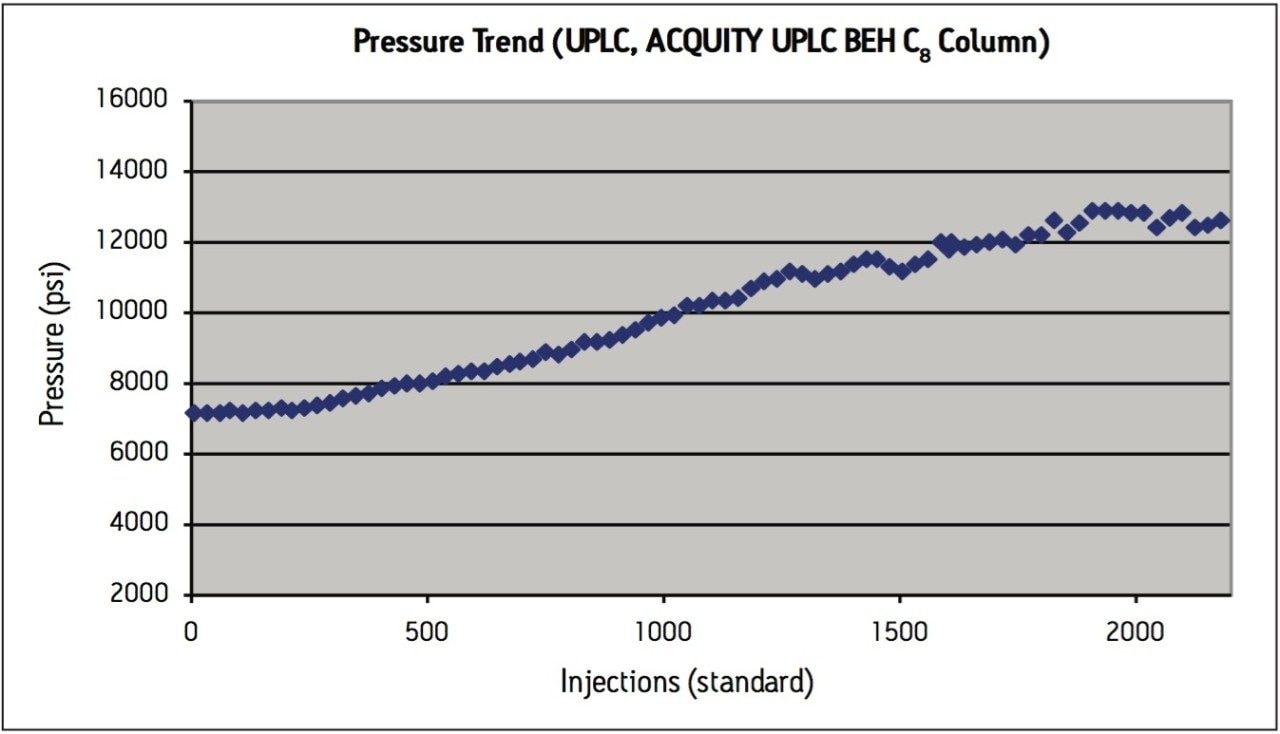
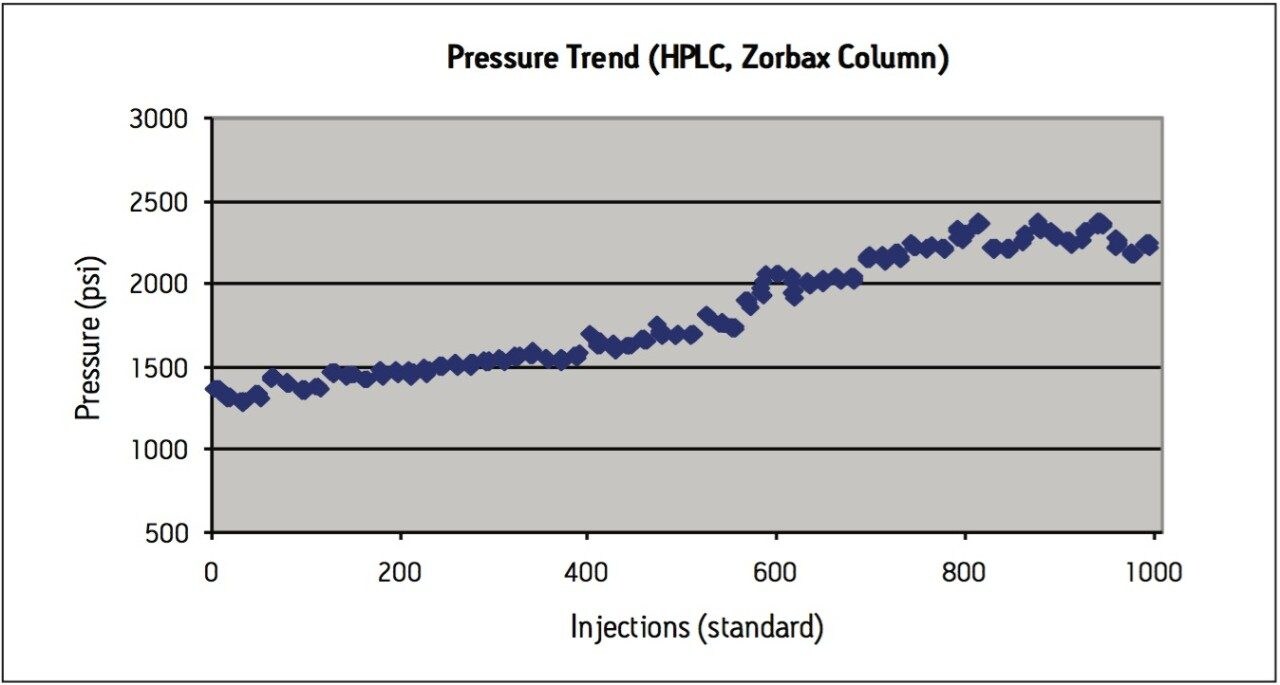
The routine use evaluation on the ACQUITY UPLC system was stopped at 2200 injections. The assay suitability criteria still passed all requirements, as shown in Table 2, demonstrating robust column performance for levonorgestrel and ethinyl estradiol on the ACQUITY UPLC BEH C8 column, even after 2000 injections.
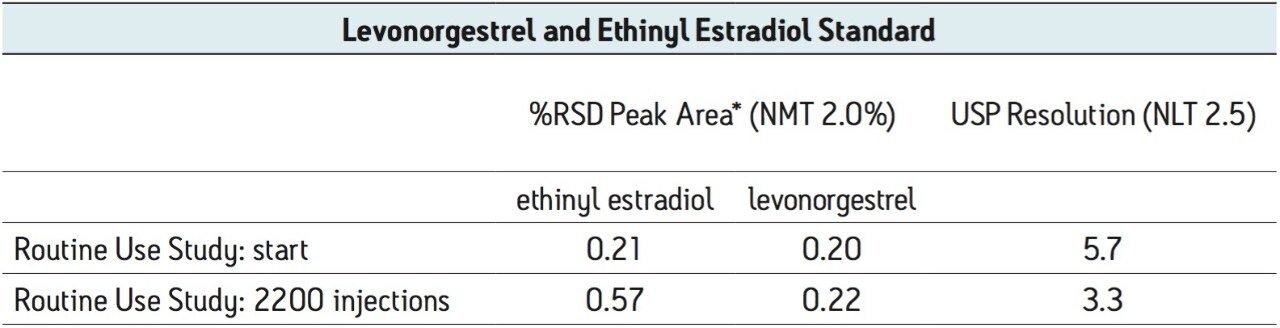
*from 5 replicate standard injections
Table 2. USP assay suitability results before and after 2200 injections from the routine use evaluation using the UPLC transferred method.
Although the UPLC method meets assay suitability criteria for more than 2000 injections, a modification to the method was made in an attempt to alleviate the pressure increase over time, which is thought to be caused by sample build–up on the column bed. A second routine use evaluation was performed on a new ACQUITY UPLC BEH C8 column, this time adding a gradient wash at the end of the isocratic USP method after each sample injection. After the 1.5 minute isocratic portion of the separation for the UPLC method, a gradient to 100% acetonitrile in 0.3 minutes was added, with a 1-minute hold at 100% acetonitrile (wash step) and then re-equilibration at 100% mobile-phase A. While the addition of a gradient wash lengthened the total cycle time to 4 minutes (including re-equilibration), this high organic washing step after each injection helps to elute the hydrophobic sample components and prevent build-up of sample on the column, thereby stabilizing the pressure throughout repeated injections from routine use (Figure 3c).
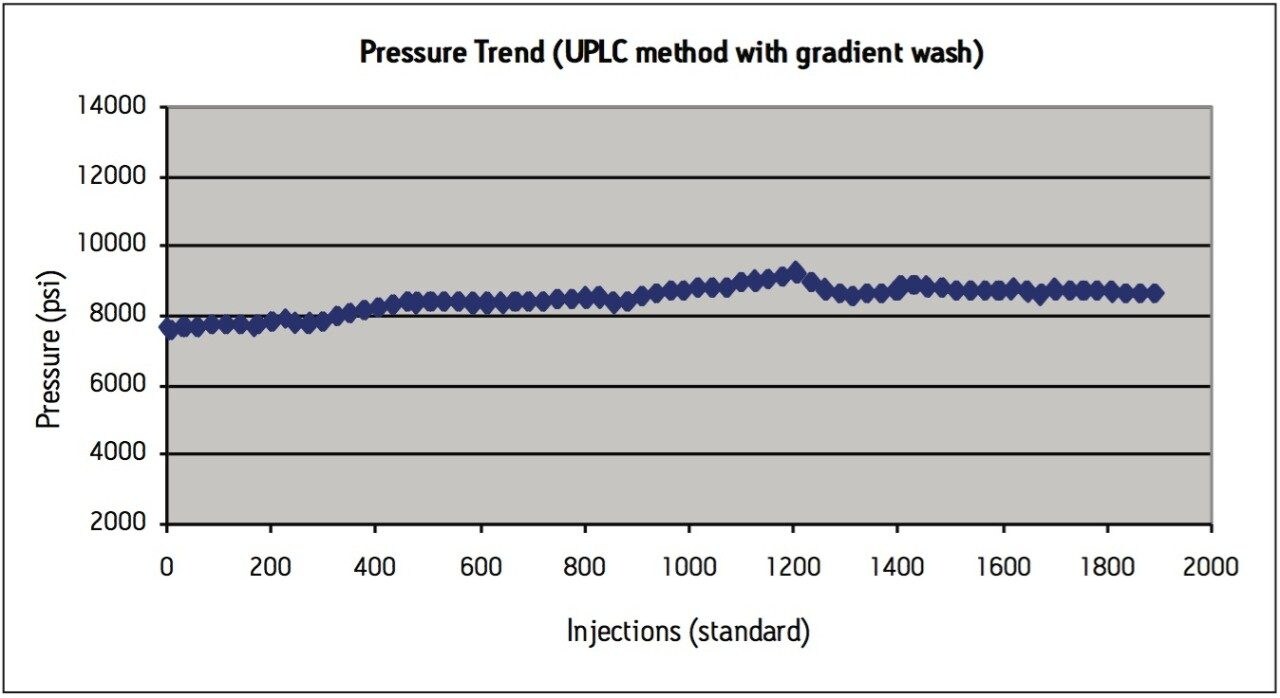
The USP compendial method for levonorgestrel and ethinyl estradiol tablets was successfully transferred from HPLC to UPLC using scalable column chemistries and the ACQUITY UPLC Columns Calculator. The UPLC method is approximately 85% faster than the HPLC method and results in a 92% savings in sample amount injected and mobile-phase solvent consumption. While extended centrifugation of the tablet sample was helpful in preparing a better quality sample for injection, it did not fully alleviate the increased pressure seen in both HPLC and UPLC during a routine use study. Instead, incorporation of a gradient wash to the isocratic method aided in preventing sample build-up on column, thereby stabilizing the pressure during routine use evaluations. Routine use of the UPLC transferred USP method was evaluated using formulated tablet samples on an ACQUITY UPLC BEH C8, 1.7 μm column. After 2200 injections, the column still passed all USP assay suitability specifications for levonorgestrel and ethinyl estradiol tablets, demonstrating that extended column performance is achievable for high throughput analysis of generic tablet formulations using an isocratic USP monograph method.
720004156, April 2013