This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the combination of ETD with nanoACQUITY UPLC coupled to SYNAPT G2. HDMS provides an alternative fragmentationtechnique for low-energy CID. This ETD method is better suited for site-specific determination of PTMs.
Obtain precise identification of the O-linked binding site using an ETD-generated mass spectrum.
Erythropoietin (EPO) stimulates the body to produce red blood cells. EPO may be used, for example, as a possible treatment to combat anemia in people who have been exposed to chemotherapeutic type drugs. EPO is a very heterogeneous glycoprotein containing both N- and O-linked glycosylated peptides and as such, is composed of a series of very closely related isoforms. Tandem MS spectra employing collision induced dissociation (CID) is usually dominated by glycan-related product ions, which hampers peptide sequence information for precise site-specific identification of important post-translational modifications (PTM).
A Waters nanoACQUITY UPLC System was utilized to perform peptide separation using a 75 μm x 200 mm column packed with 1.7 μm BEH 130 C18 particles. Peptides were eluted using a gradient from 1% to 40% acetonitrile containing 0.1% formic acid over 30 minutes at a flow rate of 300 nL/min. The eluent was directed to the NanoFlow ion surce of the mass spectrometer. Approximately 250 fmol of recombinant EPO was injected onto the column during the analysis.
The employment of CID, as shown in Figure 1, offers little information on the determination of peptide sequence and the precise site-specific identification of O-glycosylation. During the analysis, the acquisition was switched out of MS survey mode and CID was performed on the doubly-charged tryptic peptide ion m/z 1061 EAISPPDAA(S)*AAPLR modified with HexNAc-Hex-NeuAc. In brief, CID spectra of selected N- or O-linked glycopeptides precursor ions for structural elucidation purpose are usually dominated by glycan-related product ions. As a consequence, this provides little peptide sequence information for precise site-specific PTM identification.
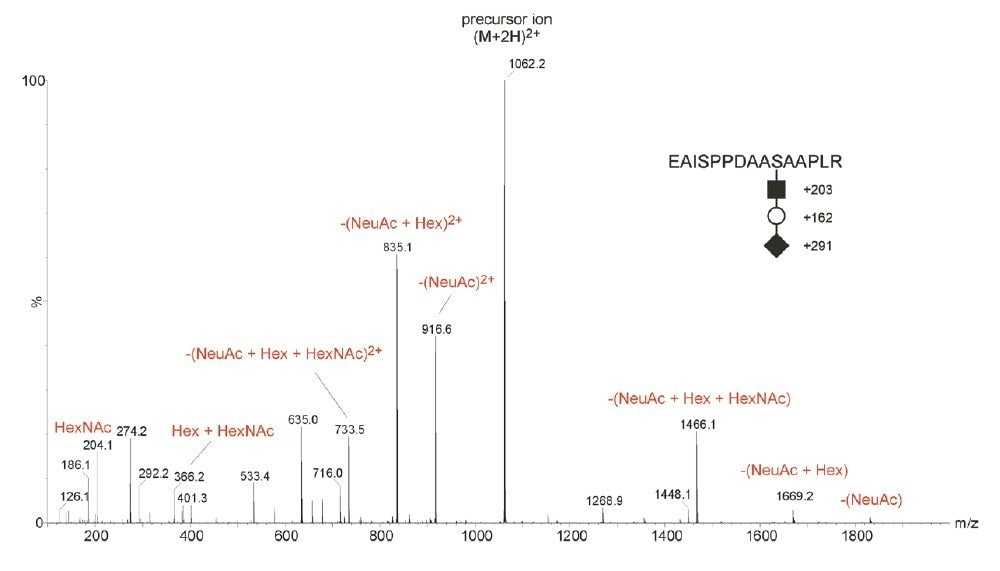
ETD is a powerful fragmentation technique that is particularly useful for determining modification sites of labile PTMs, which are often difficult to characterize by the CID approach, as shown in Figure 1. ETD is a radical-driven fragmentation technique and results in cleavage of the peptide N-Cα bond to give c and z• peptide fragment ions. During the analysis, the acquisition was switched out of MS survey mode and ETD was performed, as shown in Figure 2, on the triply-charged tryptic peptide ion of m/z 710.
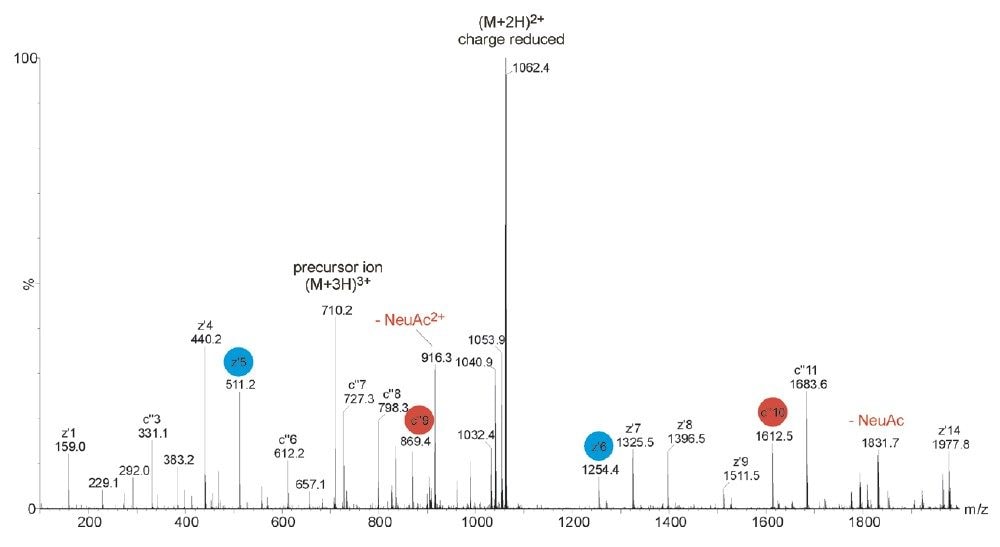
The glycosylated peptide of interest contained three proline residues. Due to the cyclic structure of proline, N-terminal dissociation adjacent to this residue is not observed using ETD. Therefore even electron N-terminal c ions were only detected for c3 +, c6 +- c11 +, and c13 +- c14 +. Odd-electron, C-terminal ions were detected for z1 +•-z2 +•, z4 +•- z9 +• and z12 +•- z14 +•. The mass difference between c9 + and c10 + together with z5 +•- z6 +• clearly showed the mass of the glycosylated serine residue, modified with HexNAc-Hex-NeuAc. Thus, in contrast to the CID mass spectrum, the ETD-generated mass spectrum provided precise identification of the O-linked binding site.
It has been demonstrated that the combination of nanoACQUITY UPLC with SYNAPT G2 HDMS and the implementation of ETD as an alternative fragmentation technique to low-energy CID is better suited for the site-specific determination of especially labile PTMs, in this case O-linked glycosylation of EPO.
720004160, December 2011