
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to rapidly analyze 24 carcinogenic aromatic amines at legislated levels using Waters ACQUITY UPLC H-Class System, coupled with the ACQUITY SQ Detector, and Empower 3 Software.
Screen samples for carcinogenic aromatic amines 7 times faster than conventional HPLC methods.
Azo dyes are used in a wide variety of consumer goods, including leather, clothing, food, toys, medical devices, plastics, and cosmetics. There are more than 3,000 azo dyes that are available in a broad spectrum of colors, and these represent more than 65% of the global dye market.
Some azo dyes can degrade and release the carcinogenic aromatic amines listed in Table 1. The potential health risk of carcinogenic aromatic amine exposure to consumers has led to stricter government regulations worldwide. U.S. FDA regulations 21 CFR 74.705 and 21 CFR 74.706 restrict the use of azo food dyes that could degrade into carcinogenic aromatic amines. EU Directive (2002/72/EC) prohibits the use of food contact materials that release carcinogenic amines. EU Directive (2002/61/EC) bans the use of azo dyes in textile and leather articles, which, upon reduction, form carcinogenic aromatic amines.
Screen samples for carcinogenic aromatic amines 7 times faster than conventional HPLC methods. The ACQUITY UPLC H-Class System, coupled with the ACQUITY SQ Detector and Empower 3 Software, is an LC-MS system that is specifically designed to comply with governmental regulations and QC protocols required by manufacturers and retailers. Using mass spectrometry to improve selectivity, the system is able to monitor multiple SIR channels, thereby tracking peak position, even with co-eluting peaks. This enables a reduction in method development time and provides confidence in the detection of specific analytes. The system performance is automatically monitored, insuring high data quality and more efficient use of the analyst’s time.
Empower 3 Software chromatography data system (CDS) provides advanced data storage and security management, and other enhanced features, including audit trails to manage compliance with 21 CFR Part 11 and other GxP requirements.
In compliance with global regulations, and in order to offer consumers safer products, major textile and leather product manufacturers and retailers have issued product certifications and QC protocols that limit the maximum amount of carcinogenic aromatic amines to no more than 20 mg/kg. To be more cost effective, there are growing demands for faster and more accurate analytical methods to test for these aromatic amines in consumer products.
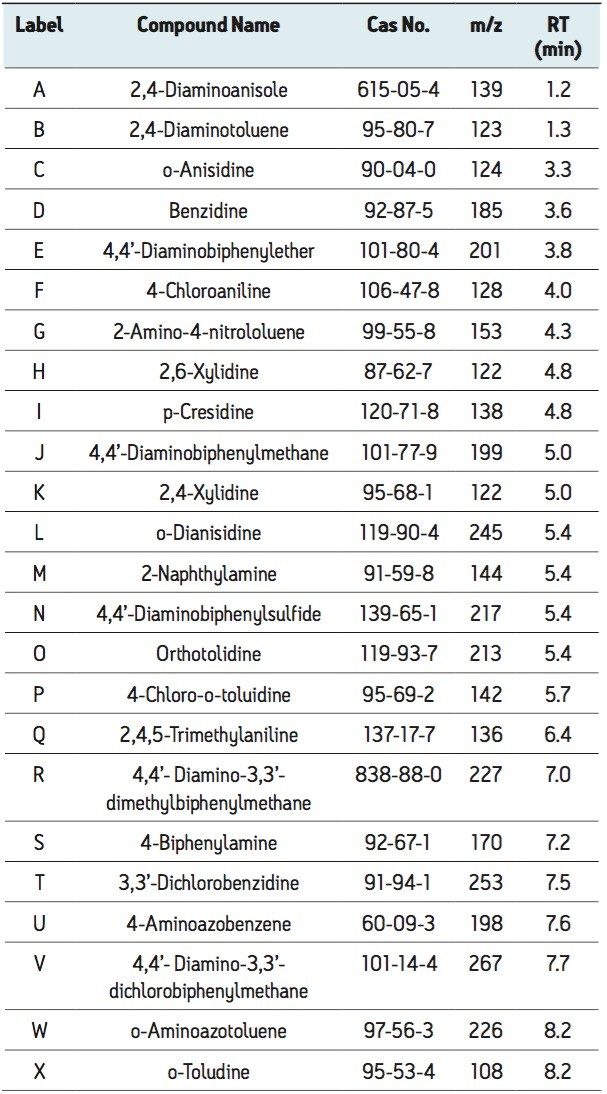
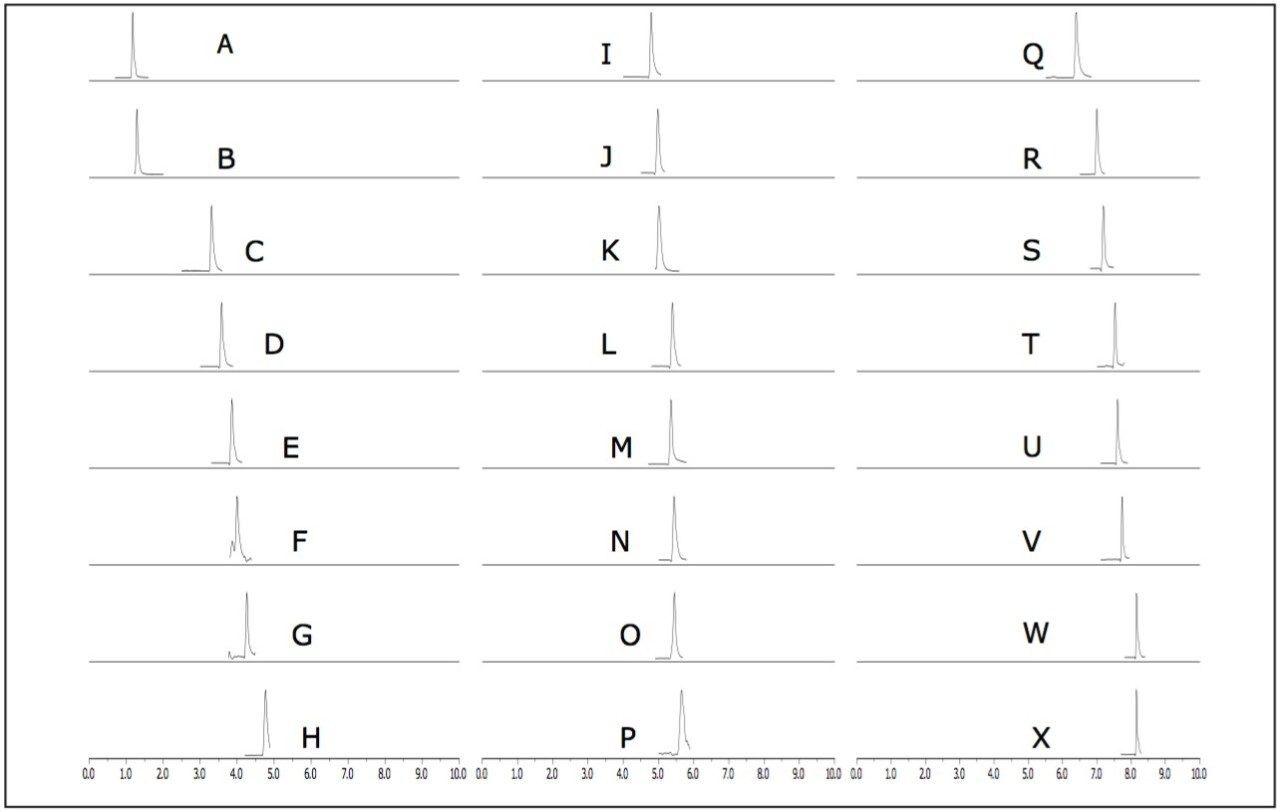
Using the ACQUITY UPLC H-Class System with the ACQUITY SQD, 24 carcinogenic aromatic amines were analyzed in 10 minutes using SIR mode. The SIR chromatograms shown in Figure 1 indicate that the carcinogenic aromatic amines were all easily detected at 20 times below the legislative limit. The structural isomers 2,6-xylidine (G) and 2,4-xylidine (J) were well separated, with retention times of 4.8 and 5.0, respectively.
Compared with the conventional HPLC/PDA method1, which typically requires 70 minutes for the separation of carcinogenic aromatic amines, this solution requires only 10 minutes, greatly increasing sample throughput. The ACQUITY UPLC H-Class/SQD System can be easily added to laboratories that already use Empower Software, circumventing the requirement of dedicated MS software. Empower users can thereby reap the benefits of MS without the need for additional training.
This work illustrates that the ACQUITY UPLC H-Class System, combined with ACQUITY SQD and Empower 3 Software enables rapid, selective, sensitive, and reproducible analysis of 24 carcinogenic aromatic amines. This 10-minute LC-MS method can be used to screen for the presence of carcinogenic aromatic amines in samples at the regulated limits. The ability to quickly and unambiguously screen samples for carcinogenic aromatic amines can facilitate quality control and regulatory compliance in textile and leather related industries. In addition, with a separation that is seven times faster than the conventional HPLC method, solvent consumption is reduced, and less hazardous waste is generated, resulting in cost and safety benefits. Other industries that have vested interests in the analysis of carcinogenic aromatic amines can also benefit from this methodology. Examples include the cosmetics, personal care products, food, and food packaging industries.
720003728, October 2010