
This is an Application Brief and does not contain a detailed Experimental section.
This application brief successfully transfers the compendial HPLC method for the analysis of powdered soy isoflavones extract to a UPLC method to achieve a faster solution that maintains data quality.
Using the ACQUITY UPLC H-Class System, a USP compendial HPLC method was successfully transferred to UPLC. The new UPLC method was approximately five times faster. When high-quality results are produced more quickly, laboratory productivity increases while cost-per-sample decreases.
The biological effects of soy isoflavones are a topic of considerable interest. These compounds are thought to reduce menopausal effects in women and provide prostate health benefits in men. Scientific evidence suggests that the anticarcinogenic properties of the isoflavone genistein are due to its action as an estrogen antagonist.
Dietary supplement manufacturers routinely use HPLC to analyze soy and soy extracts for isoflavone content. The current U.S. Pharmacopeia (USP) compendial method uses a long, shallow gradient that takes 74 minutes per injection. This long run time limits the ability of manufactures to release products quickly. In addition, a simple sample set run consisting of a blank, five calibration standards, and two retention time check solutions requires more than 10 hours before even running the first sample.
The benefits of analyzing isoflavones using a faster solution that maintains data quality – linearity, retention time reproducibility, and resolution between compounds of interest and other components in the sample – are as clear as they are plentiful: improved productivity, increased revenues, enhanced efficiency, faster sample turnover, and reduced labor and training costs.
Two different powdered soy isoflavone extracts were analyzed using the current HPLC compendial method, as written, using the ACQUITY UPLC H-Class System. A five-point standard curve was generated for each of the target compounds (daidzin, glycitin, genistin, daidzein, glycitein, genistein) with R2 values greater than 0.999 for all compounds. Retention time reproducibility for all compounds in samples was less than 0.30% RSD. Resolution between the two most closelyeluting compounds of interest (daidzin and glycitin) was 5.6. Total amounts of isofavones in the two samples were 38.9 mg/g and 25.5 mg/g, respectively.
Using the ACQUITY UPLC Columns Calculator, the HPLC method was transferred to UPLC employing an ACQUITY UPLC HSS T3 Column, 2.1 x 100 mm, 1.8 μm. The geometrically scaled method had a run time of 24.3 minutes. Because the scaled flow rate of 0.319 mL/min is below the optimum linear velocity of the sub-2-μm particle column, the gradient was rescaled to 0.60 mL/min, a flow rate closer to optimum.
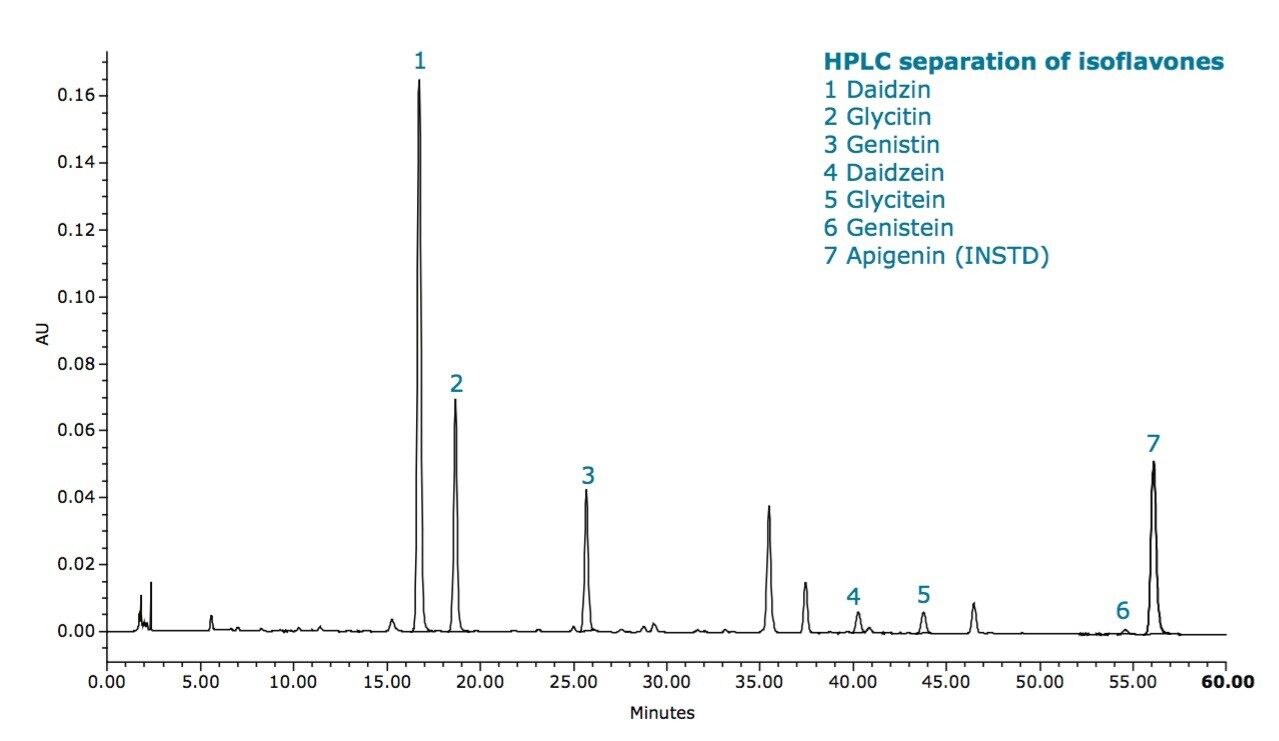
Since the ACQUITY UPLC H-Class System can routinely operate at pressures up to 15,000 psi (1,000 bar) and has low system band-spreading, sub-2-μm particle columns can be operated at their optimum linear velocity to yield highly efficient separations in a much shorter time. Using this optimal flow rate, a total run time of 15.7 minutes was achieved – a reduction of close to five times compared to HPLC (Figure 2).
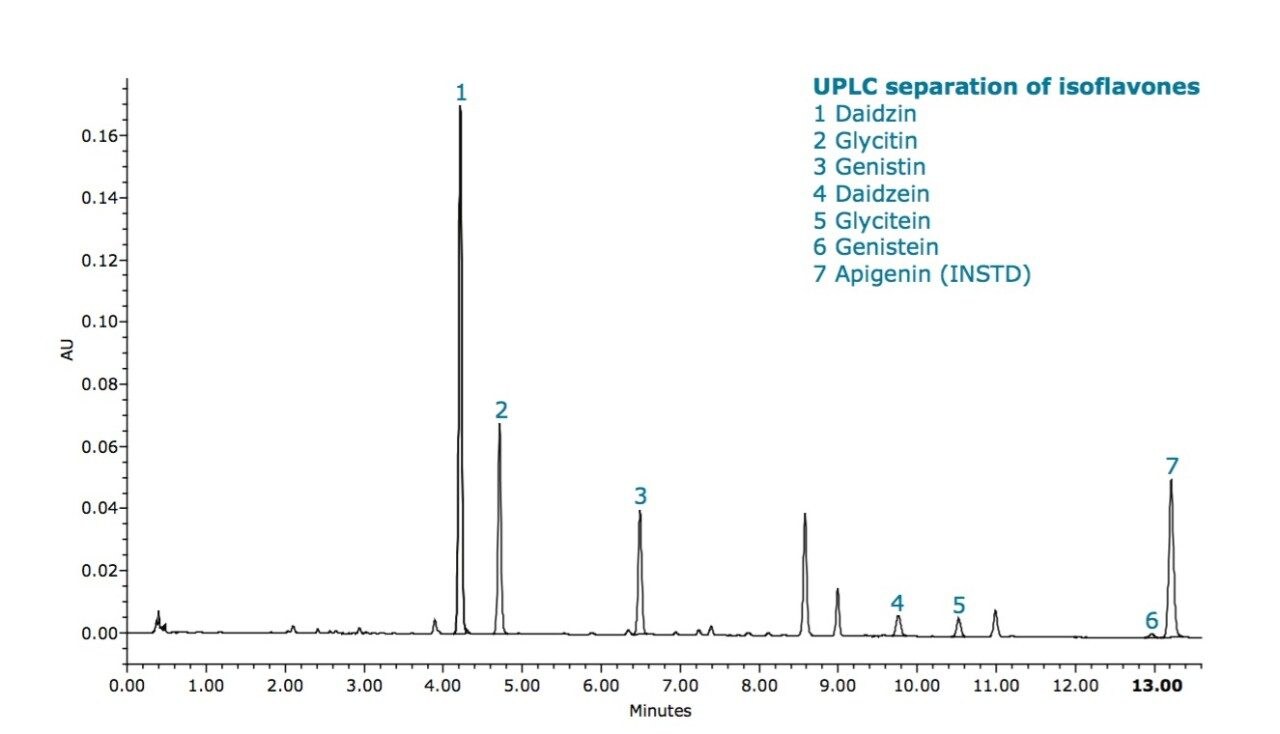
Analytical results using this new and significantly faster UPLC method were not compromised in quality, with R2 values greater than 0.999 for all compounds, retention time reproducibility for all compounds in samples was less than 0.20% RSD, and resolution between the two most closely eluting compounds (daidzin and glycitin) increasing to 6.4. Total amounts of isofavones in the two samples were in good agreement with the HPLC method, giving 38.8 mg/g and 25.1 mg/g, respectively.
Using the ACQUITY UPLC H-Class System, a USP compendial HPLC method was successfully transferred to UPLC. With the help of the ACQUITY UPLC Columns Calculator, the transfer was easy to accomplish. The new UPLC method was approximately five times faster and produced data of equal or better quality than the current HPLC method. When high-quality results are produced more quickly, laboratory productivity increases while cost-per-sample decreases. The ACQUITY UPLC H-Class System is an ideal solution for laboratories that seek to improve operational capacity by moving current HPLC methods to more efficient and cost-effective UPLC methods.
720003284, January 2010