
This application note describes an alternative HPLC method for analysis of 13 carbonyl compounds. The method achieves the desired detection limits outlined in the California EPA method 430, as well as those specified in the US EPA methods (TO-11A
Analyzing for the presence of low molecular weight aldehydes and ketones (carbonyl compounds), especially in ambient air, has significant economic and social impact. In part, this is due to their effects on humans, especially irritation of the mucous membranes, eyes, upper respiratory tract, and skin. Aldehydes can also cause injury to plants. Formaldehyde is the most common of these compounds, due to its role in the formation of photochemical ozone1. Finally, many of the carbonyl compounds are primary and/or secondary air pollutants.
Carbonyl compounds can be formed in several ways including; (i) natural occurrence, (ii) through production of chemicals, rubber, paper, etc., (iii) as secondary pollutants formed in the atmosphere, and (iv) through mobile combustion sources. Therefore, this is a major application area for the automotive industry, especially in California where emission standards are the most stringent2. Failure to meet these standards translates to increased cost, poor output efficiency, and decreased productivity.
Perhaps the most common method for analyzing these pollutants by HPLC is in their derivatized form. Typically, a known volume of sample (e.g., ambient air) is drawn through a cartridge containing acidified DNPH (2,4-dinitrophenylhydrazine)3. Common air samplers such as Waters Sep-Pak DNPH-Silica and XPoSure cartridges trap aldehydes and ketones and immediately react them with DNPH to form stable hydrazone derivatives4,5. The cartridges are then washed with 100% acetonitrile (CH3CN) to elute all of the derivatized carbonyl compounds for subsequent HPLC analysis. The approved methods for analyzing these compounds involve mobile phases that contain a large amount of CH3CN. While it provides the best separation, CH3CN is becoming a scarce and costly commodity, thus alternative methods are desired.
This application note describes an alternative HPLC method for analysis of 13 carbonyl compounds. The method achieves the desired detection limits outlined in the California EPA method 430, as well as those specified in the US EPA methods (TO-11Aand 8315A). Only 10% CH3CN is used in the elution solvent, resulting in an 86 to 96% reduction in the amount of CH3CN used when compared to the methods mentioned above. This translates to more than a 20-fold reduction in solvent cost per HPLC run, which is equivalent to thousands of dollars in solvent savings over time.
SunFire C18 columns are high-purity-based silica columns that provide unique selectivity for the separation of DNPH-derivatized aldehydes and ketones. Due to their state-of-the-art bonding and end-capping processes, SunFire C18 columns experience little secondary interactions with analytes due to low residual silanol activity. Their high loadability and best-in-class peak shape are ideal for applications where lower detection limits are required.
|
System: |
Waters Alliance 2695 Separations Module equipped with a 2998 PDA detector |
|
Data System: |
Empower 2 software (Build 2154) |
|
Column: |
SunFire C18, 4.6 x 250 mm, 5 μm (P/N 186002560) |
|
Mobile Phase A: |
10/90 CH3OH/H2O |
|
Mobile Phase B: |
60/30/10 CH3OH/THF/CH3CN |
|
Flow-rate: |
1.5 mL/min |
|
Gradient: |
56-80% B in 15 min, to 100% B in 1 min, hold for 2 min, reset (22 min total run time) |
|
Column Temperature: |
40 °C |
|
Injection Volume: |
20 μL |
|
Detection: |
365 nm, 2 Hz sampling rate, normal filter time constant |
Aldehyde and ketone standards derivatized with DNPH were supplied from the manufacturer at a concentration of 100 μg/mL (100 ppm) in 100% CH3CN. Subsequent mixtures of all 13 compounds were prepared from these stock solutions down to the 10 ppb level in 100% CH3CN.
Chromatograms demonstrating the separation of all 13 carbonyl compounds are shown in Figure 1. A solvent blank is also shown for comparison. Adequate separation of all 13 peaks is achieved in under 15 minutes with a total run time of 22 minutes. This run time is very similar to that in the isocratic method specified in CA EPA method 430, and 3x faster than that in the gradient method specified in US EPA methods TO-11A and 8315A. Based on the signal-to-noise (S/N) ratios for the 50 ppb standard shown in Figure 1, calculated limits of quantitation (LOQ, S/N = 10) for the method are between 10 and 20 ppb, with limits of detection (LOD, S/N = 3) between 2 and 5 ppb. These values are well below the lowest calibration standard (100 ppb) specified in CA EPA method 430.
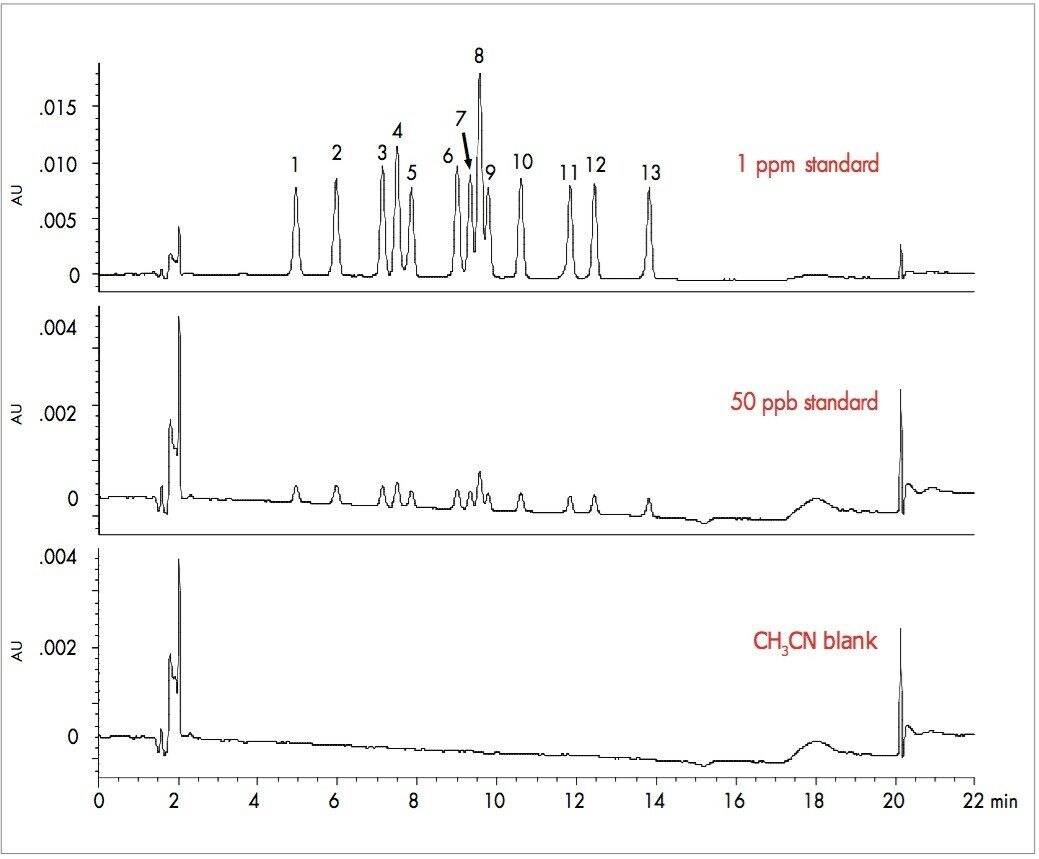
Table 1 shows a comparison of the method developed on the SunFire C18 column with the two US EPA methods and the CA EPA method in regard to analysis time and solvent cost.
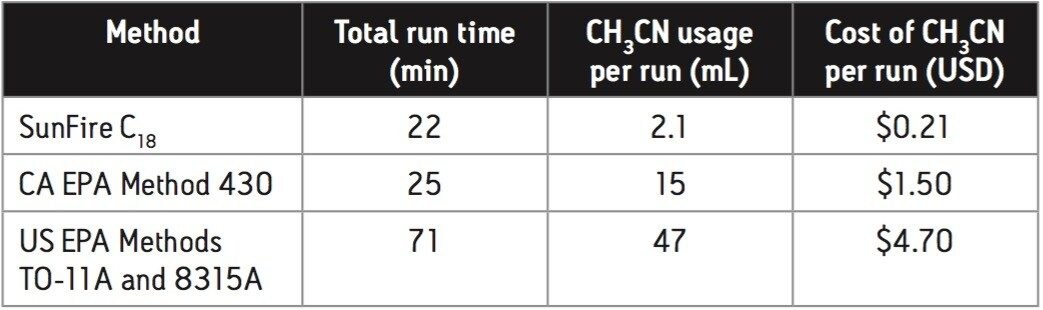
The cost benefits of using the newly developed SunFire C18 method are readily apparent.. This method uses approximately 7–fold less CH3CN than the CA EPA method (same run time) and reduces the CH3CN consumption in the US EPA methods by greater than 20–fold. In addition, the SunFire C18 method’s run time is 3x faster than that of the US EPA methods.
The SunFire C18 method costs between 86% and 96% less per run– in terms of CH3CN usage–than the other methods listed in Table 1. For an HPLC system that is used 8 hours per day, 5 days per week, 50 weeks per year, this translates to a $6,800 savings in CH3CN cost alone for the SunFire C18 method.
An HPLC method was developed on a SunFire C18 column in order to minimize the use of CH3CN for the analysis of aldehyde and ketone pollutants. The run time is similar to that specified in the CA EPA method 430 but uses up to 22–fold less CH3CN than current methods. This translates into significant solvent cost reductions (86 to 96%). The lower limit of quantitation is well below the limits needed for accurate quantitation in ambient air. The method developed here can be used in conjunction with Waters Sep-Pak DNPH-Silica and XPoSure cartridges for measuring aldehyde and ketone pollutants in atmospheric and ambient air samples, including automobile emissions.
720003012, April 2009