
In this application note, some principles of transferring methods between UPLC and HPLC systems are illustrated. In particular, calculations required to move a method from a UPLC to an HPLC platform are presented.
Over the past 5 years, UPLC Technology has been widely adopted by analytical laboratories due to the benefits of increased sample throughput, better separation efficiency, and improved data quality over traditional HPLC. As these laboratories replace their existing HPLC systems with UPLC systems, there is a transition period where a method must be run on both platforms. Thus, having the same particle substrate and bonded phases available in HPLC and UPLC particle sizes can significantly ease the burden of method development and transfer from one platform to another.
In addition to the ethylene bridged hybrid (BEH) particles, three new high strength silica (HSS) substrate stationary phases for HPLC-have been introduced. In this application note, some principles of transferring methods between UPLC and HPLC systems are illustrated. In particular, calculations required to move a method from a UPLC to an HPLC platform are presented.
|
Systems: |
UPLC - ACQUITY UPLC and PDA HPLC - Alliance 2695 and 2998 PDA |
|
Column Chemistries: |
ACQUITY UPLC HSS C18 ACQUITY UPLC HSS C18 SB ACQUITY UPLC HSS T3 |
|
Column Dimensions: |
UPLC - 2.1 x 50 mm, 1.8 µm HPLC - 4.6 x 100 mm, 3.5 µm HPLC - 4.6 x 150 mm, 5 µm Semi-prep - 10 x 50 mm, 5 µm |
|
Mobile Phase A: |
0.1% TFA in water |
|
Mobile Phase B: |
0.1% TFA in acetonitrile |
|
Sampling Rate: |
UPLC - 20 Hz HPLC - 5 Hz |
|
Time Constant: |
UPLC - 0.1 s HPLC - 0.4 s |
All conditions for Figure 1 are listed in the figure caption. For Figures 2 through 5, the gradient, flow rate, injection volume, column temperature, and detection wavelength are indicated within each figure.
Scaling the injection volume
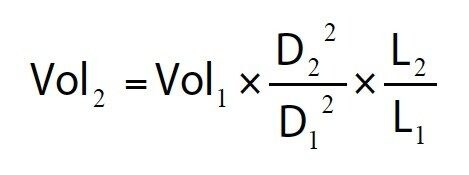
Where Vol is the injection volume (µL), D is the column inner diameter (mm), and L is the column length (mm). For example, a 2.1 μL injection on a 2.1 x 50 mm UPLC column is equivalent to a 30 μL injection on a 4.6 x 150 mm HPLC column.
Scaling the flow rate
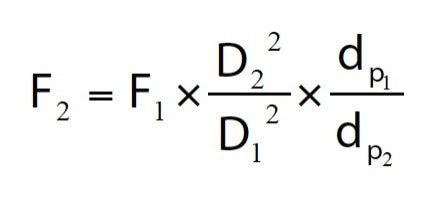
Where F is the flow rate (mL/min) and dp is the particle size (μm). For example, a 0.6 mL/min flow rate on a 2.1 mm i.d., 1.8 μm column corresponds to a flow rate of 1.04 mL/min on a 4.6 mm i.d., 5.0 μm column.
The dwell volume difference between the ACQUITY UPLC System and HPLC systems will result in retention time shifts when transferring from UPLC to HPLC. In order to achieve proper transfer results, this difference in dwell volume must be compensated for by introducing an initial hold time at the start of the HPLC gradient. The detailed procedure for determining system volume has been explained previously [1]. The dwell volume must be calculated as the number of column volumes (c.v.).
Dwell Volume of UPLC System:
Measured system volume = 0.11 mL (5 μL sample loop)
Volume of a 2.1 x 50 mm UPLC column (c.v.) = 0.173 mL
Dwell volume = 0.11 mL / 0.173 mL = 0.636 c.v.
The ACQUITY UPLC system has a dwell volume of 0.636 c.v.
Dwell Volume of HPLC System:
Measured system volume = 0.80 mL (100 μL sample loop)
Volume of a 4.6 x 150 mm HPLC column = 2.49 mL
Dwell volume = 0.80 mL / 2.49 mL = 0.321 c.v.
The Alliance HPLC system has a dwell volume of 0.321 c.v.
Dwell Volume Difference:
The difference in dwell volume between the UPLC and HPLC systems = 0.636 c.v. - 0.321 c.v. = 0.315 c.v.
Therefore, the initial hold volume will be: 0.315 c.v. x 2.49 mL (c.v. of the HPLC column) = 0.784 mL
Calculating Initial Hold Time:
Hold time = initial hold volume (mL)/flow rate (mL/min) = 0.784 mL/1.04 mL/min = 0.76 min
In addition to calculating the initial hold time, the number of column volumes must remain constant for each gradient segment to properly transfer from UPLC to HPLC.
The number of column volumes (#. c.v.) = [Segment time (min) x flow rate (mL/min)]/column volume (mL).
In Table 1, #. c.v. for the 1st gradient segment (2nd row) is calculated as: [2.0 min x 0.6 mL/min]/0.173 mL = 6.94 c.v.
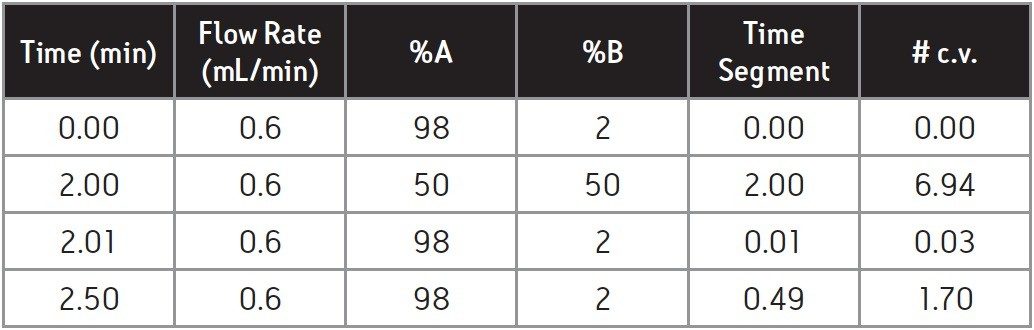
The # of column volumes (e.g., 6.94 c.v. in Table 1) must be maintained to preserve the separation profile in HPLC.
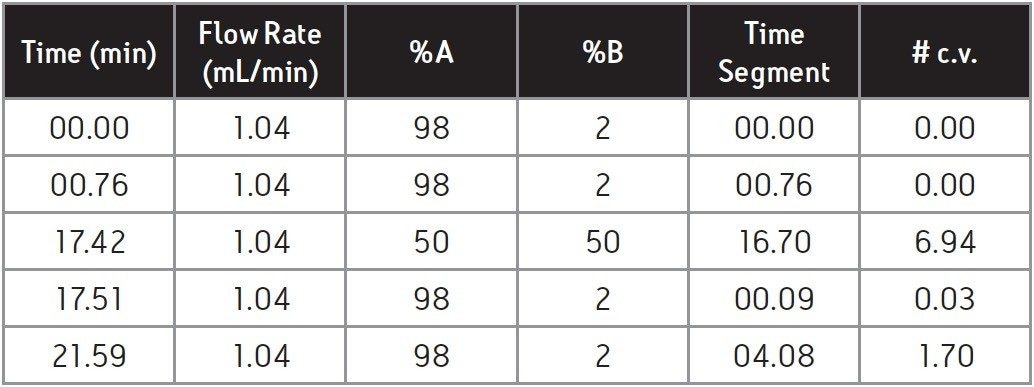
Segment time (min) = [# c.v. x column volume (mL)]/flow rate (mL/min) = 6.94 x 2.49 mL / 1.04 mL/min = 16.7 min, as show in Table 2.
By following the same calculations for all of the gradient segments and adding the initial hold time, the gradient table for HPLC can be completed (Table 2).
The HSS family of columns include a general purpose C18 (HSS C18), a non-end-capped C18 column designed to give different selectivity for basic compounds (HSS C18 SB), and a column designed to retain polar compounds and operate in 100% aqueous conditions (HSS T3). Figure 1 shows the selectivity difference between these 3 columns for a mixture of basic drugs.
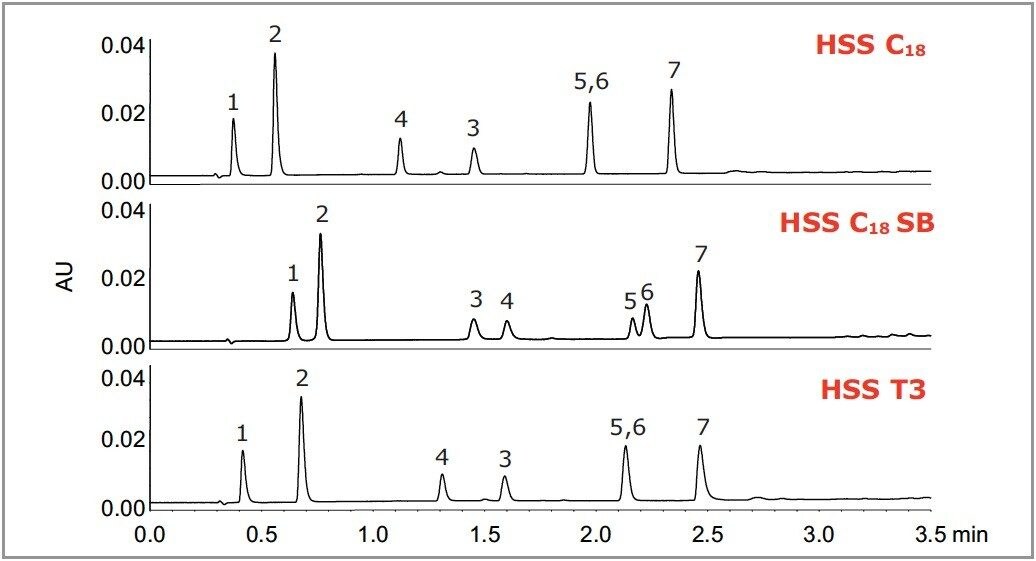
The columns used for method transfer were chosen based on their ratio of column length to particle size (L/dp), which is a measure of resolving power. In order to maintain the same resolution during method transfer between UPLC and HPLC, the following columns were used:
UPLC: 50 mm / 1.8 μm (L/dp = 27,777)
HPLC: 100 mm / 3.5 μm (L/dp = 28,571)
HPLC: 150 mm / 5.0 μm (L/dp = 30,000)
The following section shows the transfer of three different UPLC methods to HPLC using the formulas described in the Experimental section. The use of each HSS chemistry is highlighted to demonstrate consistent selectivity and resolution between UPLC and HPLC.
Glimepiride, the active pharmaceutical ingredient (API) in Amaryl (used for treating diabetes), was degraded and then analyzed by UPLC on an ACQUITY UPLC HSS C18 Column. The UPLC method (including injection volume, flow rate and gradient condition) was then properly transferred to the HPLC system on an HSS C18 Column. Selectivity, UV response, and the degradation profile are consistent between the UPLC and HPLC chromatograms.
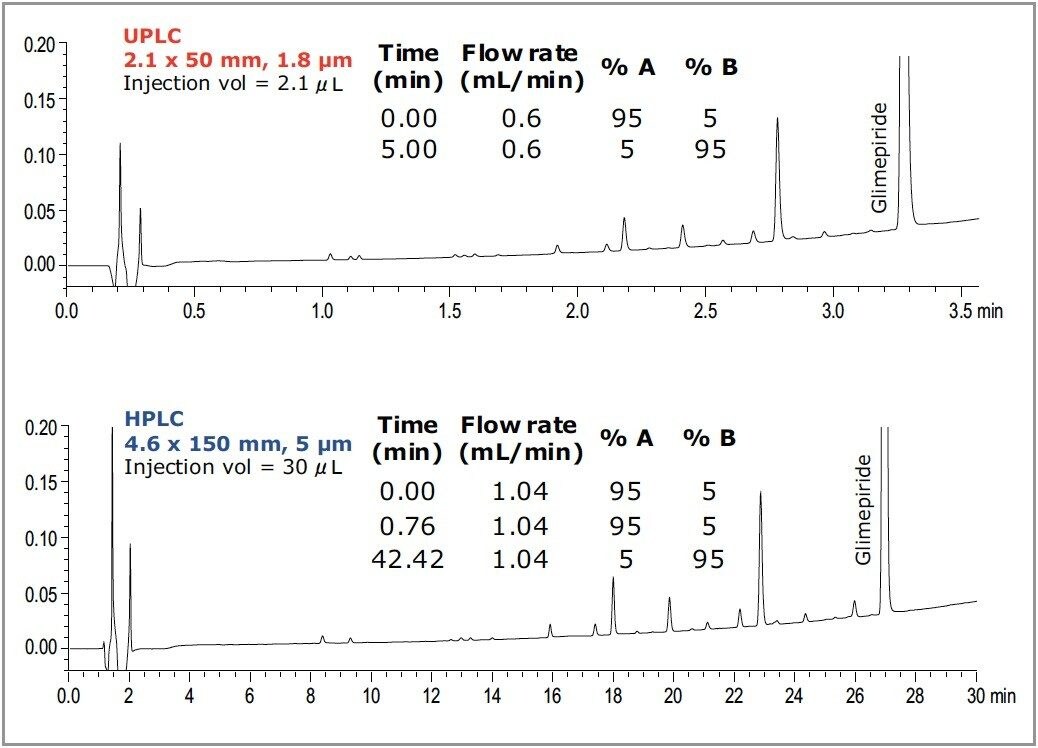
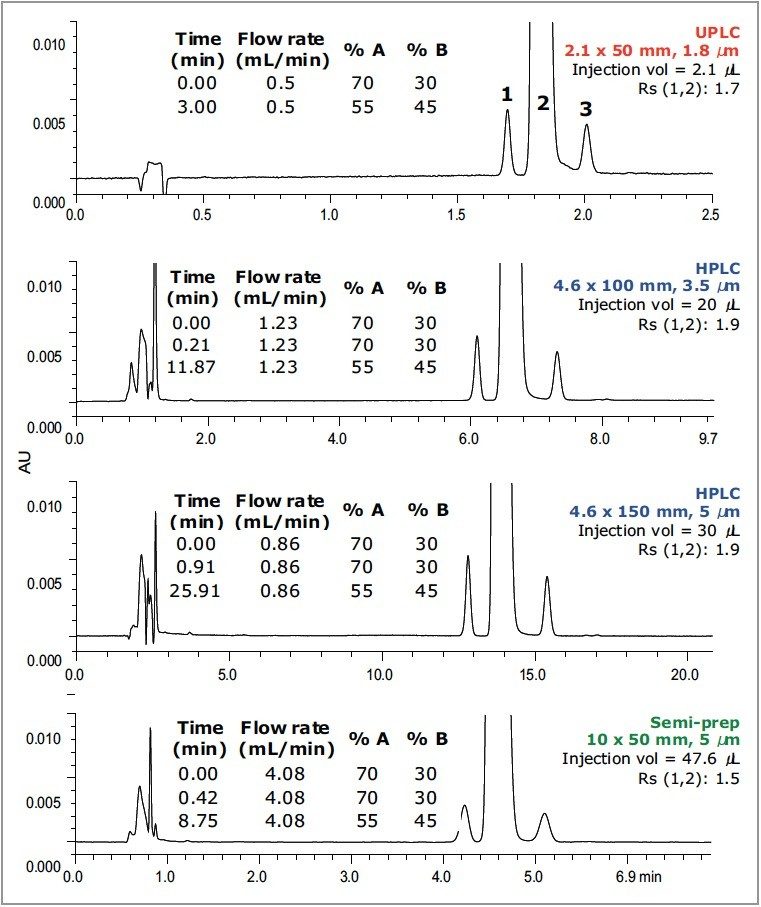
Figure 3 shows the separation profile for paroxetine and two related compounds (i.e., impurities/degradants). Paroxetine is the API in Paxil, which is used for treating depression. The separation was developed on an ACQUITY UPLC System using an ACQUITY UPLC HSS C18 SB Column, and then transferred to HPLC and semi-prep dimensions. Resolution and selectivity were maintained across all columns, proving that separations on HSS packings can be easily transferred between UPLC, analytical and preparative HPLC columns. The resolution on the 10 x 50 mm, 5 μm preparative column is slightly less than for the UPLC and HPLC separations because the L/dp ratio was lowered. This was done to shorten the preparative HPLC run time.
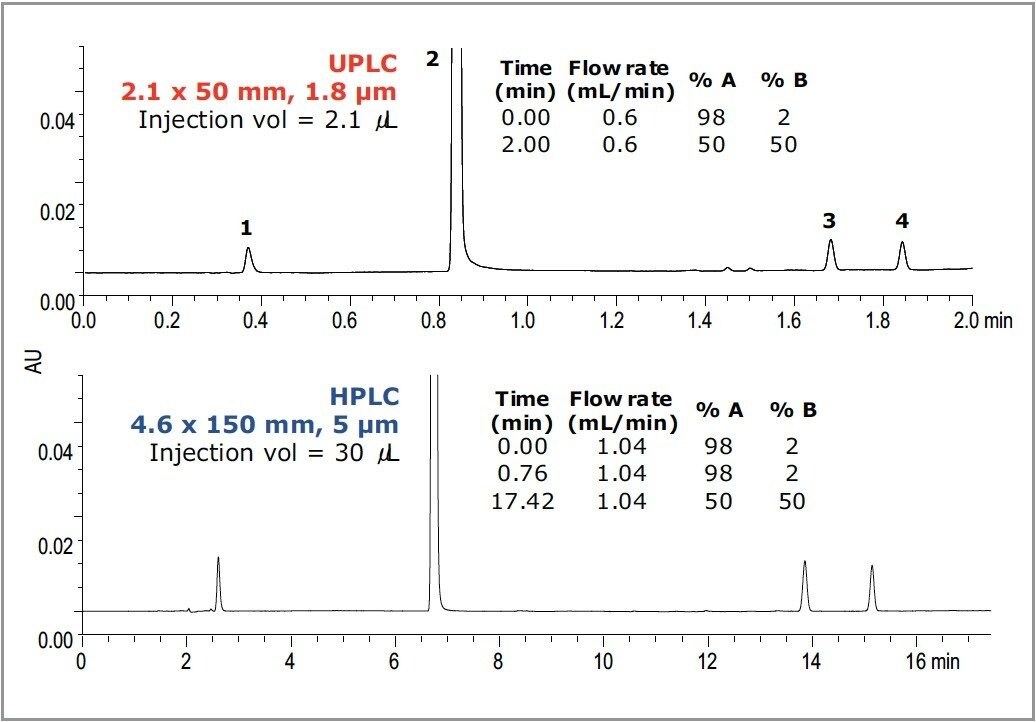
The last example shown in Figure 4 is acetaminophen, the API in Tylenol. Acetaminophen was separated from 3 of its impurities on an ACQUITY UPLC HSS T3 Column using an ACQUITY UPLC System. The UPLC method was then readily transferred to an HPLC system with an HPLC HSS T3 Column. Selectivity and resolution are comparable between the UPLC and HPLC analyses.
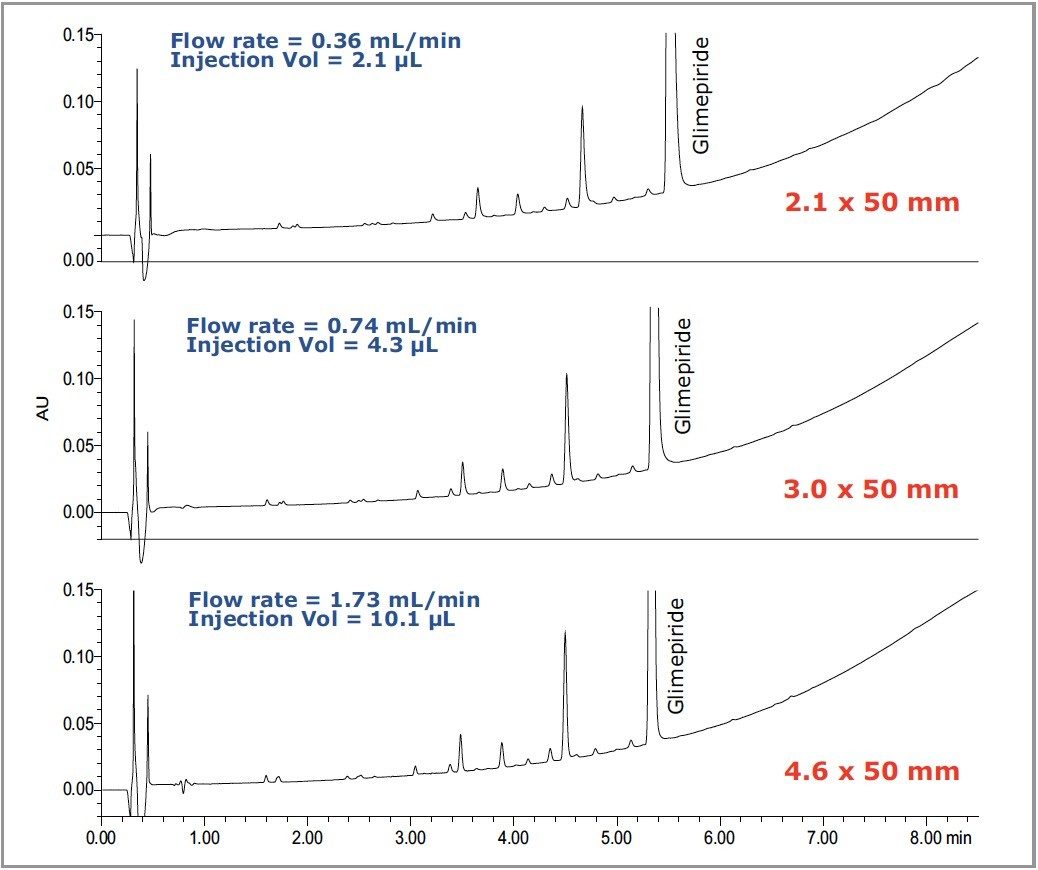
Transferring methods from one column diameter to another is also important, mostly for saving solvent and decreasing mobile phase waste disposal costs. A smaller column diameter increases sensitivity and is a better option when sample volume is limited. Figure 5 shows how the forced degradation analysis for glimepiride, shown in Figure 2, may be readily scaled to three HSS C18 columns with different column diameters. Changing the column diameter requires only an adjustment in the injection volume and flow rate, while the gradient remains the same. Using the same particle size, the separation transfers seamlessly from one column diameter to another without a loss in resolution or change in selectivity. Also note that, at the lower flow rate and column volume, 5 times less mobile phase and sample is consumed on the 2.1-mm i.d. column when compared to the 4.6-mm i.d. column. Using the same particle and surface chemistry in a smaller diameter column significantly reduces the cost of analysis without sacrificing data quality.
A successful method transfer from UPLC to HPLC requires careful consideration of key parameters, including system volume, column dimensions, particle size, injection volume, flow rate, and gradient profile. Using the concepts and calculations presented in this application note, methods can be easily transferred between different systems (UPLC- HPLC- Prep HPLC, or vice versa), particle sizes, and column diameters using the new HSS columns.
720003233, October 2009