
This application note highlights recent advances in the chromatographic and mass spectrometric technologies via the analysis of a multi-component mixture for surveillance monitoring of pesticides in agricultural produce.
Pesticides are often used in the production of foodstuffs. The concentrations of individual pesticides permitted in our food are controlled by legislation. There is, therefore, a requirement for surveillance monitoring of pesticide residues in foodstuffs. Analytical methods developed for this purpose must achieve limits of detection at or below the Maximum Residue Limit (MRL).
Given the large number of pesticides in existence and the variety of agricultural produce available, multi-residue pesticide screening methods can offer efficiency advantages over single residue and class specific methods. However, these multi-residue methods are limited both by the chromatographic separation of the analytes and the speed of data acquisition.
Tandem quadrupole mass spectrometry is often used as a detection system due to the high selectivity offered in multiple reaction monitoring (MRM) mode, which compensates for generic sample preparation methods involving minimal sample cleanup. Due to the number of potential analytes, the mass spectrometer chosen should be able to rapidly switch both between MRM channels and between positive and negative ionization modes, thereby offering the potential to achieve greater efficiency in the analysis of multi-component mixtures. Complementing these +/– ionization mode switching capabilities in the Waters Micromass Quattro Premier Mass Spectrometer is the revolutionary Waters ACQUITY UPLC System, offering improved chromatographic resolution and shorter analysis times resulting from the use of columns packed with novel 1.7 μm stationary phase particles.1
In this work, we highlight recent advances in these chromatographic and mass spectrometric technologies via the analysis of a multi-component mixture for surveillance monitoring of pesticides in agricultural produce.
The raisin sample, Californian sun-dried seedless raisins (Thompson variety) was prepared using a procedure described below involving methanolic extraction and ChemElut cleanup, evaporation and reconstitution.2
The raisin sample was chopped to avoid loss of juice. A 5 g aliquot of the homogenized sample was transferred to a blender cup, to which 9 mL of water was added. After 10 minutes, 20 mL of methanol was added and the sample was blended for 2 minutes. 6 mL of the resultant extract was mixed with 2 mL of a solution of sodium chloride (20 g in 100 mL water). A 5 mL aliquot was then transferred to a ChemElut column containing 5 mL of diatomaceous earth. After 5 minutes, the ChemElut column was eluted with 16 mL of dichloromethane. The eluate was evaporated to dryness and the dry residue was reconstituted in 250 μL of methanol and further diluted with 1 mL of water. The final extract contained the residues of 0.5 g dry sample per mL. The extract was filtered through a 0.45 μm filter into a glass sample vial.
Blank matrix was prepared from organically grown sun-dried seedless raisins (Thompson variety) using the same extraction and cleanup procedure described above. Matrix-matched standards were prepared by spiking all analytes at 0.5, 1, 2.5, 5, 10 pg/L (equivalent to 1, 2, 5, 10, 20 μg/kg, respectively).
|
LC system: |
ACQUITY UPLC System |
|
Mobile phase A: |
MeOH/H2O (1:4 v/v) + 5 mM CH3CO2NH4 |
|
Mobile phase B: |
MeOH/H2O (9:1 v/v) + 5 mM CH3CO2NH4 |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 100 mm, 1.7 μm |
|
Flow rate: |
0.45 mL/min |
|
Injection volume: |
20 μL |
|
Column temp.: |
40 °C |
|
Time |
%B |
|---|---|
|
0 min |
0% |
|
8.5 min |
100% |
|
11.0 min |
100% |
|
11.1 min |
0% |
|
13.5 min |
0% |
|
MS system: |
Quattro Premier |
|
Ionization mode: |
ES+/ES- |
|
Capillary voltage: |
0.8 kV (+/- ionization) |
|
Gas flow: |
800 L/hr |
|
Source temp.: |
120 °C |
|
Desolvation temp.: |
400 °C |
|
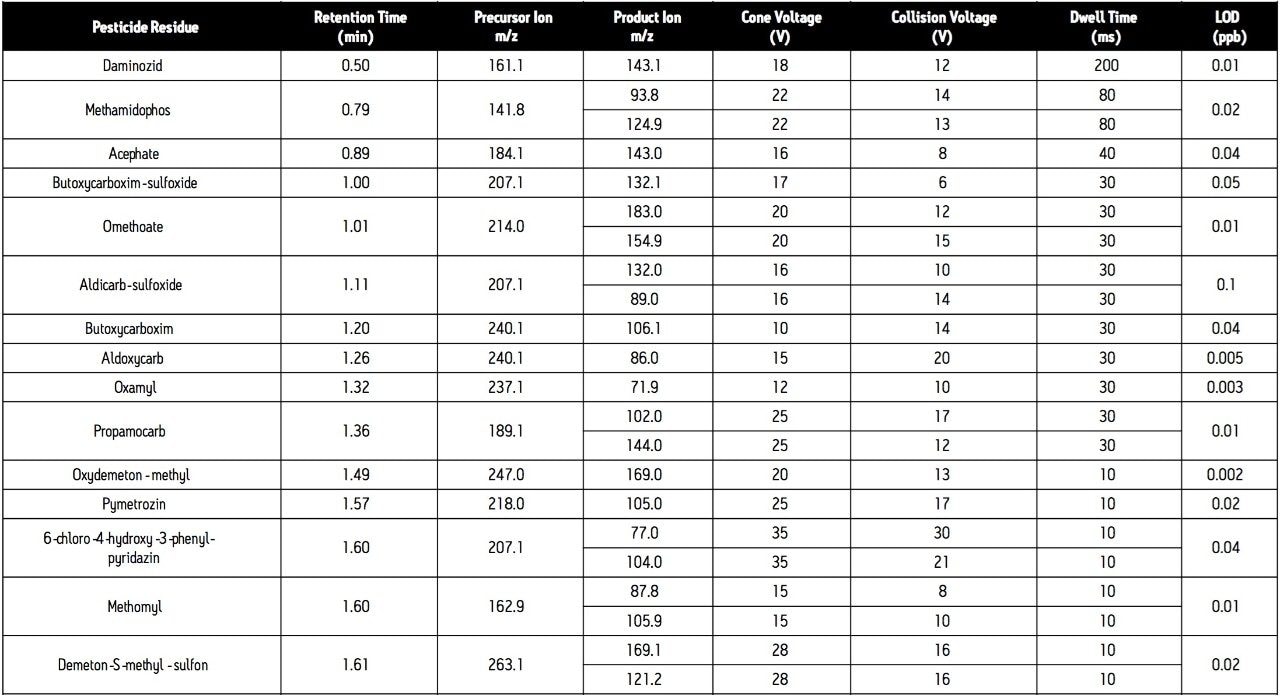
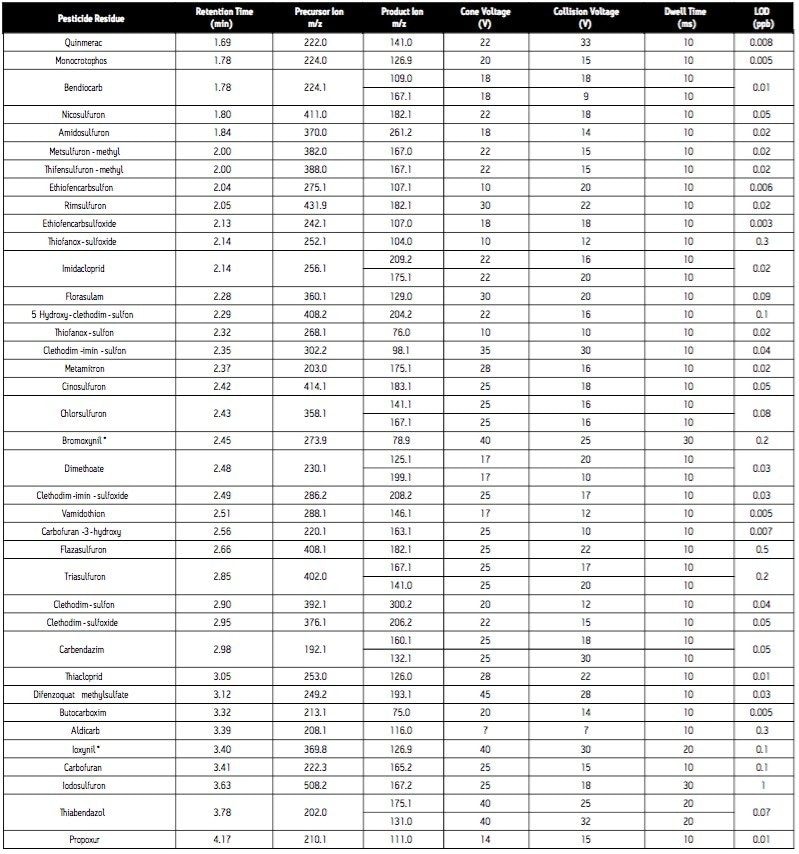
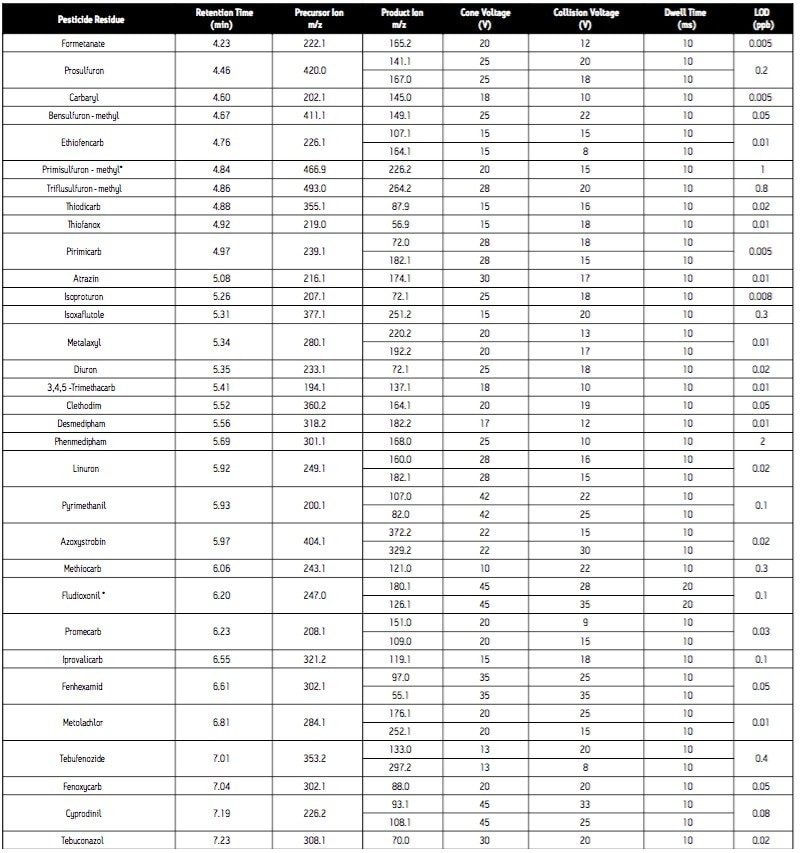
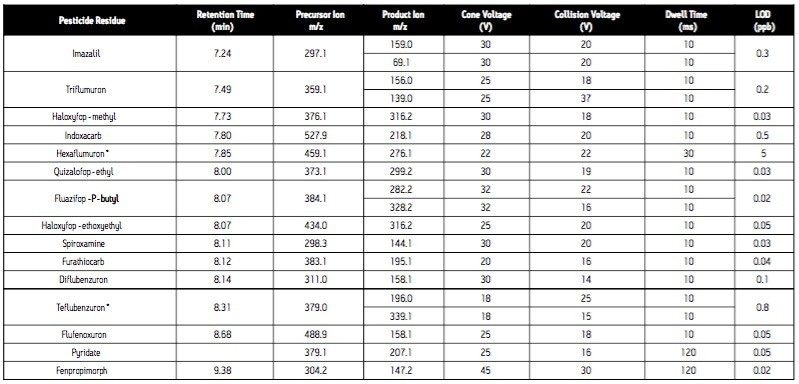
Cone voltage: |
See Table 1 |
|
MS/MS: |
Operated in MRM mode |
|
Collision voltage: |
See Table 1 |
The work details the development of a multi-residue method for the analysis of 100 pesticide residues by UPLC-MS/MS. The work is based upon a previously developed HPLC-MS/MS method using a Waters Alliance HT/Quattro Premier System, which had an overall cycle time of 25 minutes (HPLC Conditions: XTerra MS C18 Column, 2.1 x 100 mm, 3.5 μm, linear gradient from 0 to 100% B in 17 min).
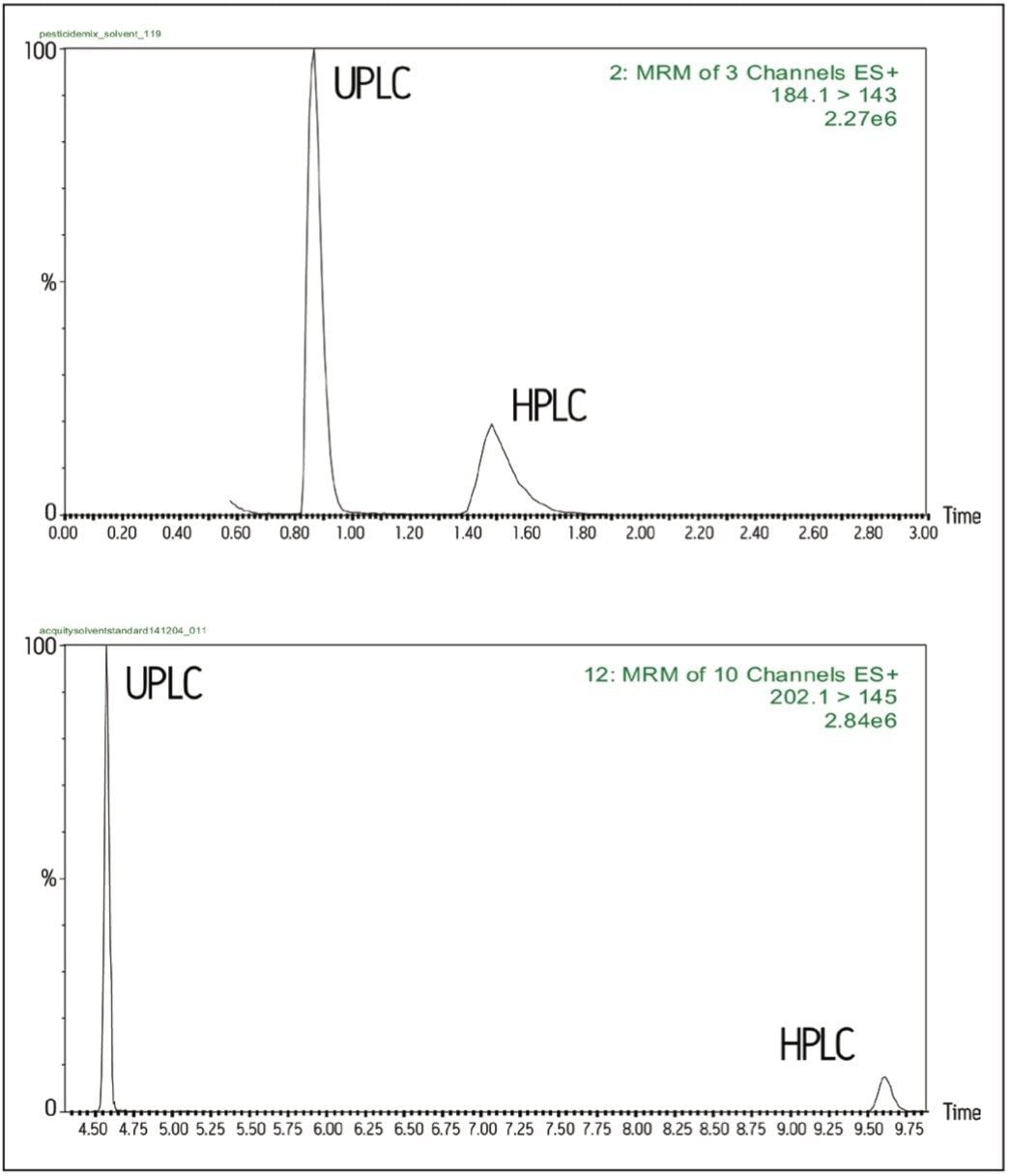
Comparison of UPLC and HPLC chromatograms is shown below (Figure 1). Peak widths observed for the majority of pesticide residues analyzed under UPLC conditions are approximately 0.1 min (cf 0.3 min under HPLC conditions). The narrower peak widths often resulted in an increase in signal response over that achieved under HPLC-MS/MS conditions (Figure 1).
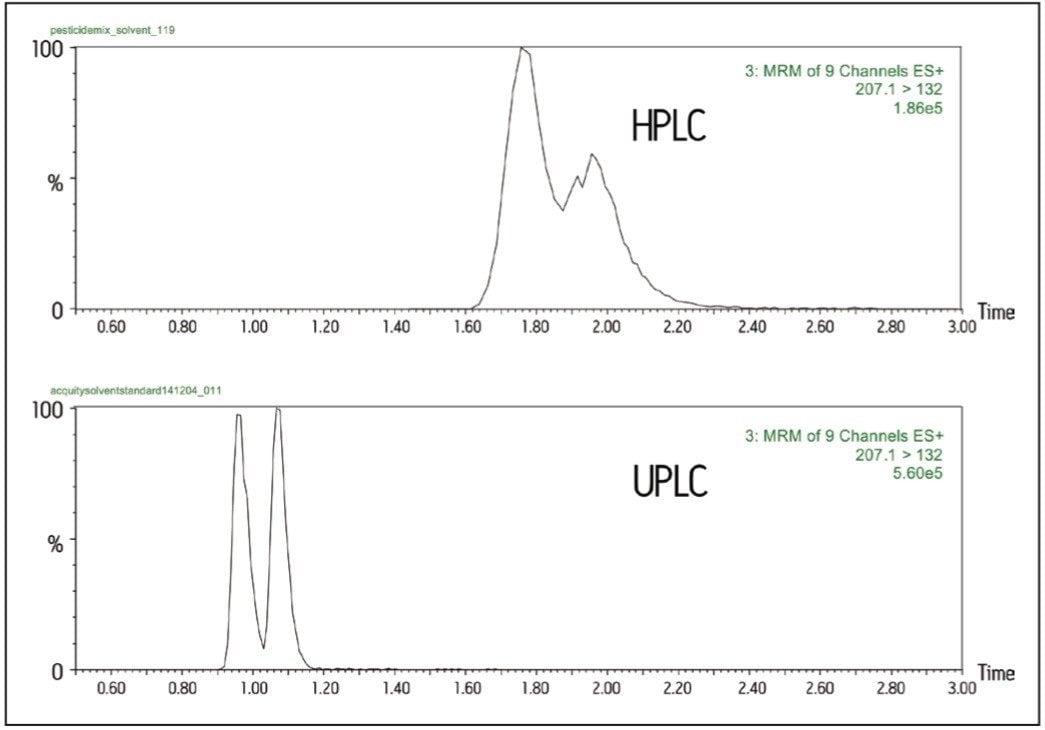
Greater chromatographic resolution is achievable under UPLC conditions (cf. HPLC) and is illustrated in Figure 2. Butoxycarboxim sulfoxide and aldicarb sulfoxide have similar retention properties, with butoxycarboxim sulfoxide eluting first, and the same MRM transition (m/z 207.1>132).
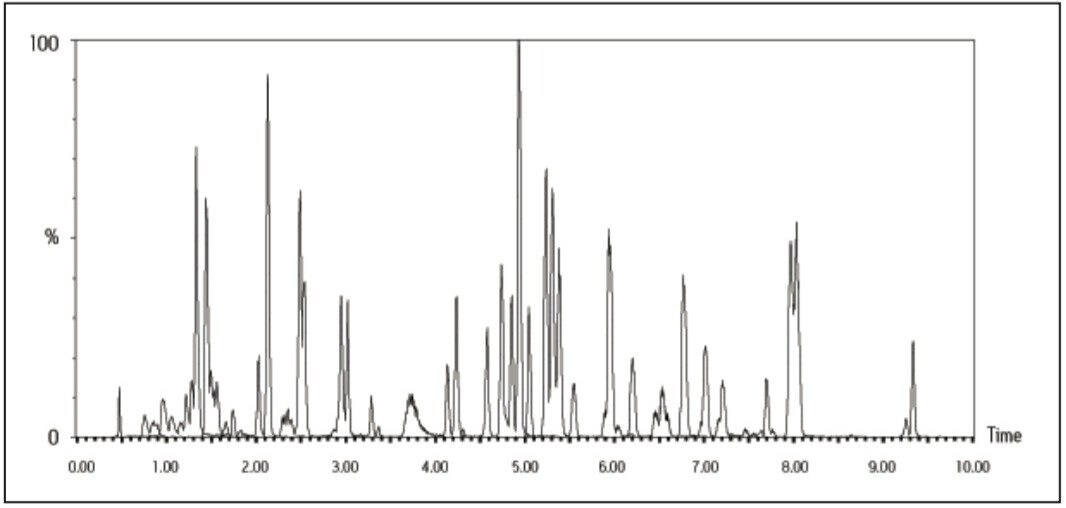
It can be seen that UPLC has the ability to separate complex mixtures. This is confirmed by considering the analysis of 100 pesticide residues in raisin matrix (Figure 3). All 100 pesticides elute within 10 minutes, and the overall cycle time is just 13.5 minutes.
Since the analytical method is intended for surveillance monitoring, it needs to be able to detect tens of pesticide residues; some of which are better detected under negative ES conditions (Table 1). The use of the ACQUITY UPLC System places added demands on the mass spectrometer due to the improved chromatographic resolution and short analysis times. For these reasons, the Quattro Premier Tandem Quadrupole Mass Spectrometer was selected as the detector for this application.
In order for accurate quantization to be performed, a minimum of 10 data points across each peak must be acquired. This requirement, coupled with the number of target analytes and narrow chromatographic UPLC peaks indicated that it would be advantageous if the MRM functions were arranged into time windows, based on analyte retention times (Figure 4). This system enabled the flexible use of dwell times (Table 1), such that those peaks with lower intensities can have their S/N ratios increased by employing longer dwell times, while retaining a minimal scan time.
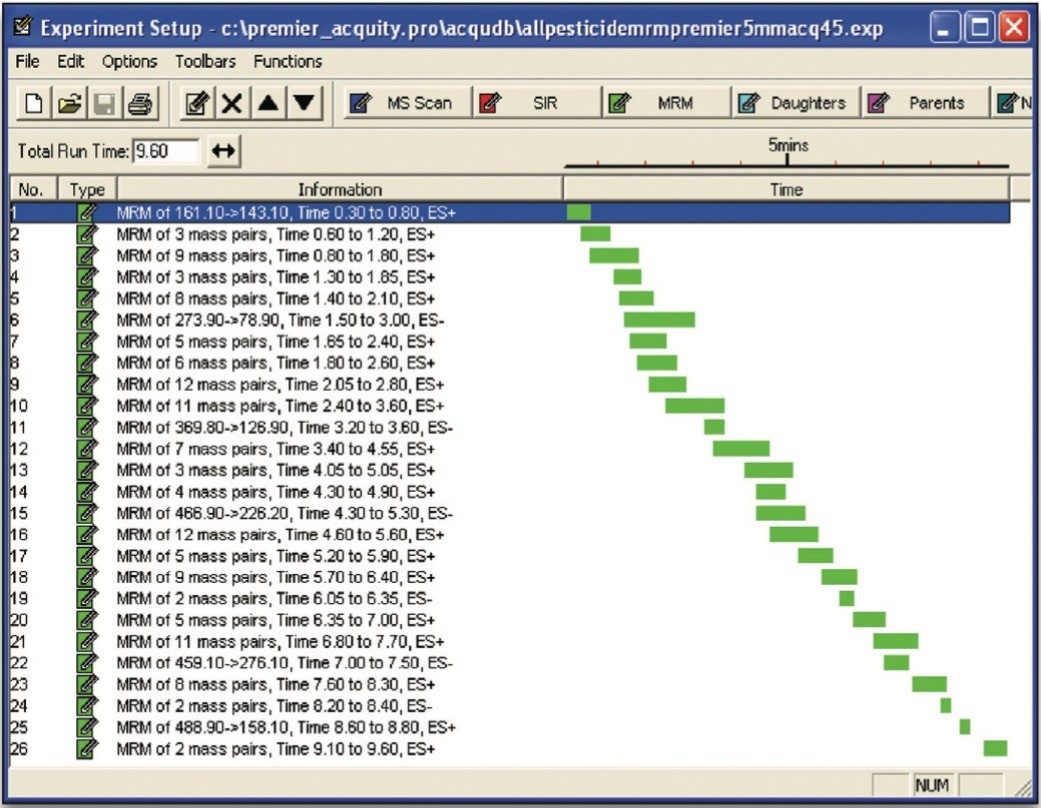
In addition to the primary MRM traces monitored for each analyte, confirmation MRM traces were incorporated into the method for the 31 most commonly found residues. In total, 131 MRM transitions were monitored in 26 time windows (Figure 4).
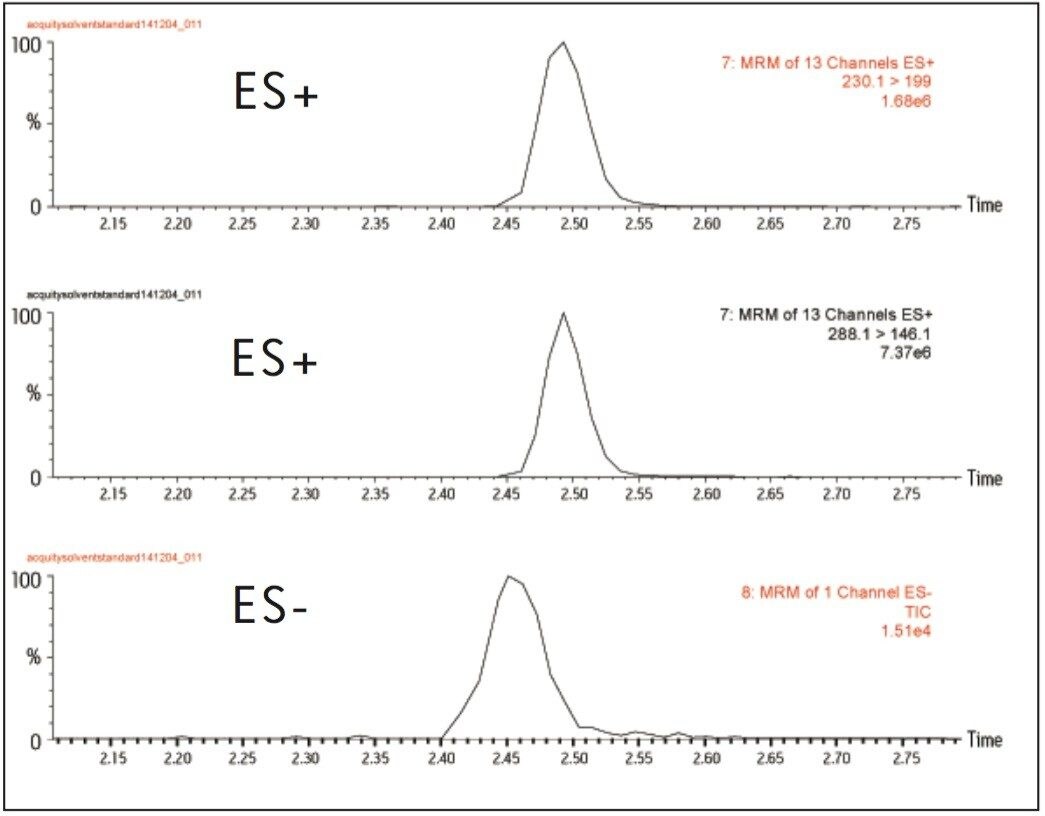
Six of the pesticides included within the method ionize under negative ES mode. The Quattro Premier can switch rapidly between positive and negative ionization modes, so that closely eluting analytes under both modes can be achieved within a single analytical run as illustrated above right (Figure 5), thereby minimizing the need to perform separate analyses.
Analysis of standard solutions enabled LODs (based on 3 x S/N) to be determined (Table 1). All are well below the necessary reporting level of individual pesticides in food (10 μg/kg, 5 pg/L), indicating that this method could be applied to the analysis of pesticide residues in a variety of matrices.
The analytical method was applied to the analysis of pesticide residues in raisins. The chromatogram (Figure 3) obtained for the analysis of a raisin sample containing the pesticides spiked at a level equivalent to the MRL demonstrates good signal response for all analytes at this reporting level. Since the analytical method is intended for surveillance monitoring, it needs to be able to detect tens of pesticide residues; some of which are better detected under ES- conditions (Table 1).
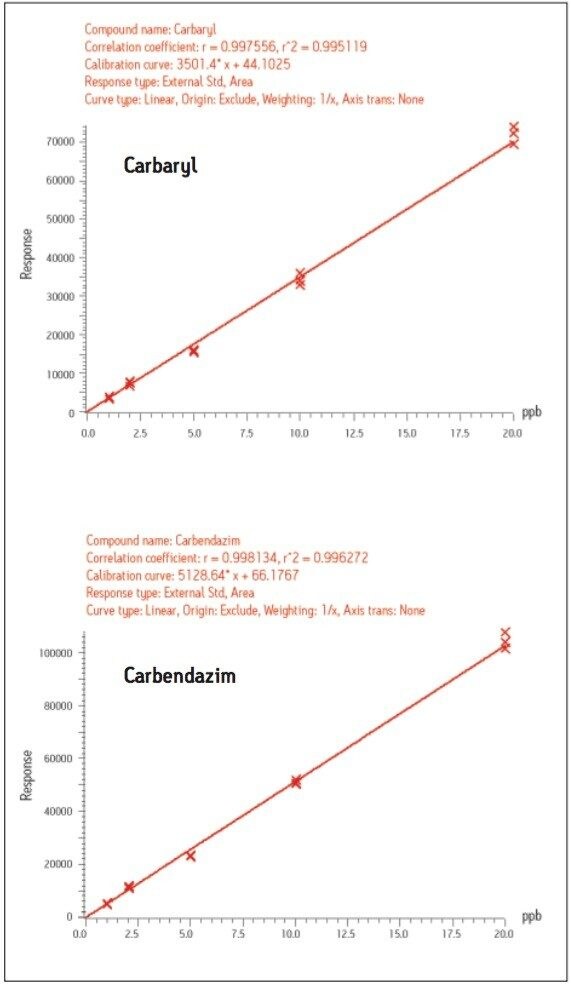
Good linearity in calibration was demonstrated over the range analyzed, 1-20 μg/kg (Figure 6).
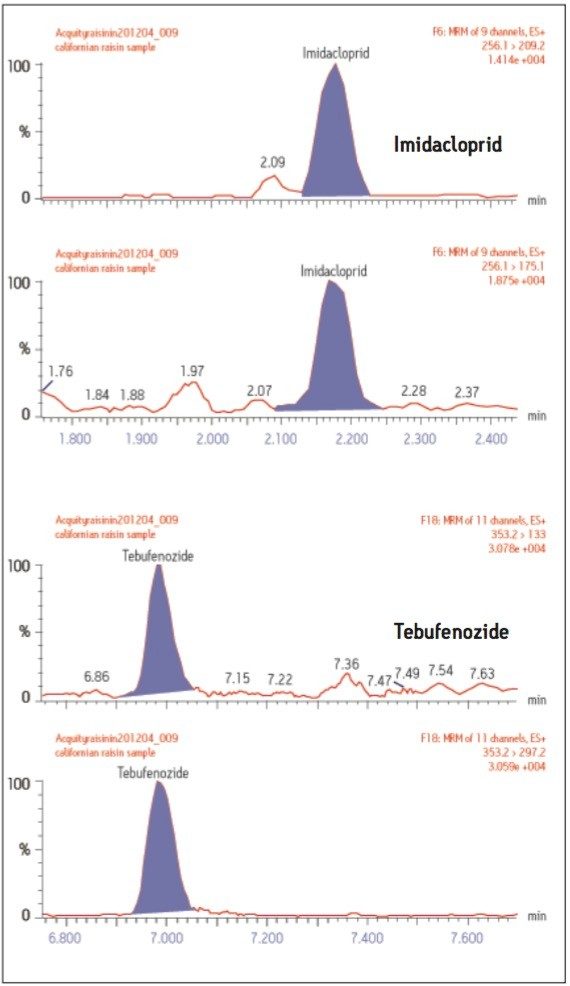
Inclusion of a second transition within the surveillance method enables unambiguous confirmation of the presence of a residue within the sample, without the need to perform a second confirmatory analytical run (Figure 7) resulting in further efficiency gains. Two pesticide residues (imidacloprid and tebufenozide) were confirmed present within the raisin sample at levels below the MRL, 4.4 and 3.4 μg/kg, respectively.
720001172, June 2007