
This application note demonstrates the advantages of UPLC-MS/MS for the quantitative analysis of five drugs in precipitated rat plasma. For comparative purposes, similar experimental protocols were performed on both UPLC and traditional HPLC instrumentation using the Quattro Premier as the MS detector.
LC-MS/MS has become the method of choice for quantitative bioanalytical applications. In the pharmaceutical industry, high throughput (HT) labs have a constant need to generate more information and to process more samples per unit time, while maintaining the quality of the data generated. To accommodate this, significant efforts were made to improve the speed and sensitivity of the liquid chromatography and mass spectrometry systems in use. UltraPerformance LC(UPLC) is a new dimension of separation science that retains the practicality and principles of HPLC separation, while increasing the attributes of speed, sensitivity and resolution.1 This is achieved by employing a new generation of LC columns that incorporates sub-2 μm hybrid packing materials, which provide the ultimate in chromatographic performance. These particles operate at high mobile phase linear velocities and generate higher back pressures. The Waters ACQUITY UPLC System has been holistically designed for low system and dwell volumes to take full advantage of the small particle technology.
MS detection benefits significantly from the performance characteristics of the UPLC technology. Increased peak concentration with reduced chromatographic dispersion will promote increased source ionization efficiency. As a result, the ACQUITY UPLC System can generate peak widths less than a second at half height (as narrow as 1.8 seconds wide at the base). For MS detection, it is generally accepted that if reproducible peak area quantification is required, the chromatographic peaks should be defined by no less than 15 data points.2 Thus, as peaks become narrower, the mass spectrometer needs to acquire data faster.
The typical data collection mode for quantification by tandem quadrupole MS is by multiple reaction monitoring (MRM). The cycle time per MRM transition is composed of two parameters: the dwell time and the interchannel delay between successive MRM transitions. The interchannel delay period allows ions to be cleared from the collision cell, preventing crosstalk. The Waters Micromass Quattro Premier Tandem Quadrupole Mass Spectrometer has been specifically designed to operate in MRM mode with the shortest of acquisition cycle times. The collision cell utilizes traveling wave (T-Wave) technology superimposed on the confining RF field to reduce ion transit time, ensuring that the cell is completely refilled before acquisition commences. This allows for very short Dwell times without a loss in signal intensity.3 As a result, the Quattro Premier is capable of MRM data monitoring with a minimum 5 ms Dwell time, a 5 ms interchannel delay, and a 5 ms interscan delay.
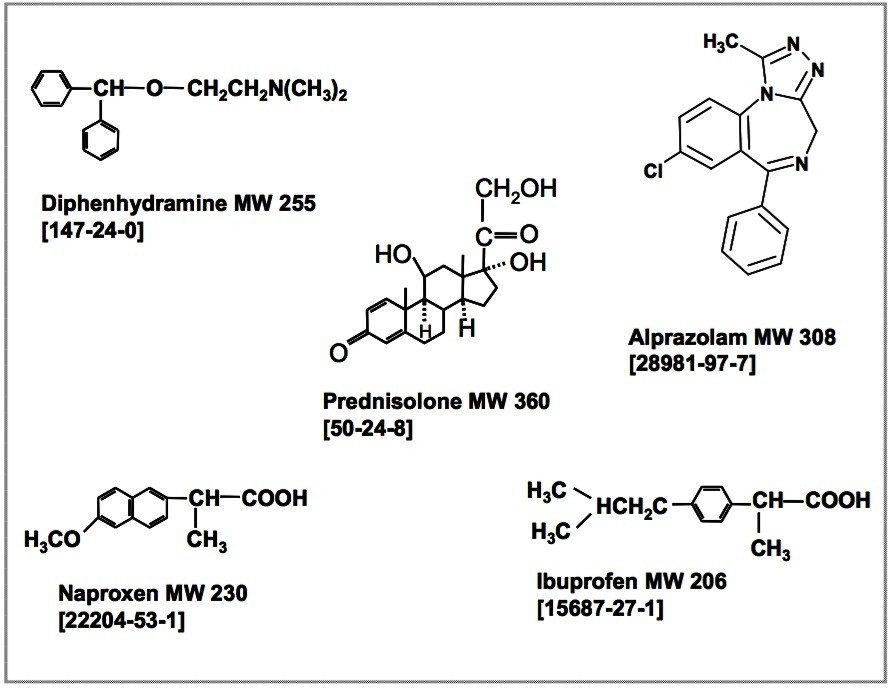

Acetonitrile was added into rat plasma at a 2:1 ratio for protein precipitation. This acetonitrile/rat plasma mixture was then voltexed and centrifuged at 4 °C and 14,000 rpm for 15 minutes. The standards were spiked into the supernatant, which was then directly injected into each of the LC-MS systems for analysis.
|
Instrument: |
ACQUITY UPLC System (UPLC) Waters Alliance HT System (HPLC) |
|
Column: |
ACQUITY UPLC BEH C18 Column, 2.1 x 50 mm, 1.7 μm (UPLC) XTerra MS RP C18 Column, 2.1 x 50 mm, 3.5 μm (HPLC) |
|
Flow rate: |
0.6 mL/minute (UPLC and HPLC) |
|
Mobile phase: |
A: 10 mM NH4OAc in 20% MeOH, pH 5.0 (UPLC and HPLC) B: 10 mM NH4OAc in 80/20 MeOH/ACN (UPLC and HPLC) |
|
Injection volume: |
5 μL (UPLC and HPLC) |
|
Time (min) |
%A |
Curve |
|---|---|---|
|
0.0 |
55 |
6 |
|
0.4 |
45 |
6 |
|
0.5 |
5 |
1 |
|
2.0 |
55 |
1 |
|
Time (min) |
%A |
Curve |
|---|---|---|
|
0.0 |
80 |
1 |
|
0.2 |
80 |
1 |
|
1.8 |
20 |
6 |
|
2.0 |
5 |
1 |
|
5.0 |
80 |
1 |
|
Instrument: |
Quattro Premier Mass Spectrometer |
|
Capillary voltage: |
0.5 kV |
|
Source temp.: |
130 °C |
|
Desolvation temp.: |
400 °C |
|
Desolvation gas: |
800 L/hr. |
|
Cone gas flow: |
50 L/hr. |
|
Data collection: |
ESI MRM (with polarity switching) |
|
Interscan delay: |
20 ms |
|
Inter channel delay: |
5 ms (UPLC), 10 ms (HPLC) |
|
Dwell times: |
ESI+ 5 ms (UPLC), 20 ms (HPLC) ESI- 20 ms (UPLC and HPLC) |
To demonstrate the high speed capability of the ACQUITY UPLC System-Quattro Premier Mass Spectrometer LC-MS/MS system, we have developed an analysis for a five drug mixture. Amongst these five compounds, three favored electrospraypositive (ESI+), and the other two favored electrospraynegative (ESI-). The Quattro Premier operated in MRM mode with polarity switching, such that all compounds were detected during a single injection.
The MRM chromatograms of the drug mixture are shown, one obtained by HPLC-MS/MS (Figure 2), the other obtained by UPLC-MS/MS (Figure 3). Each figure contains 6 MRM transitions: five for the target analytes, and one for the Alprazolaminternal standard, Alprazolam-D5.
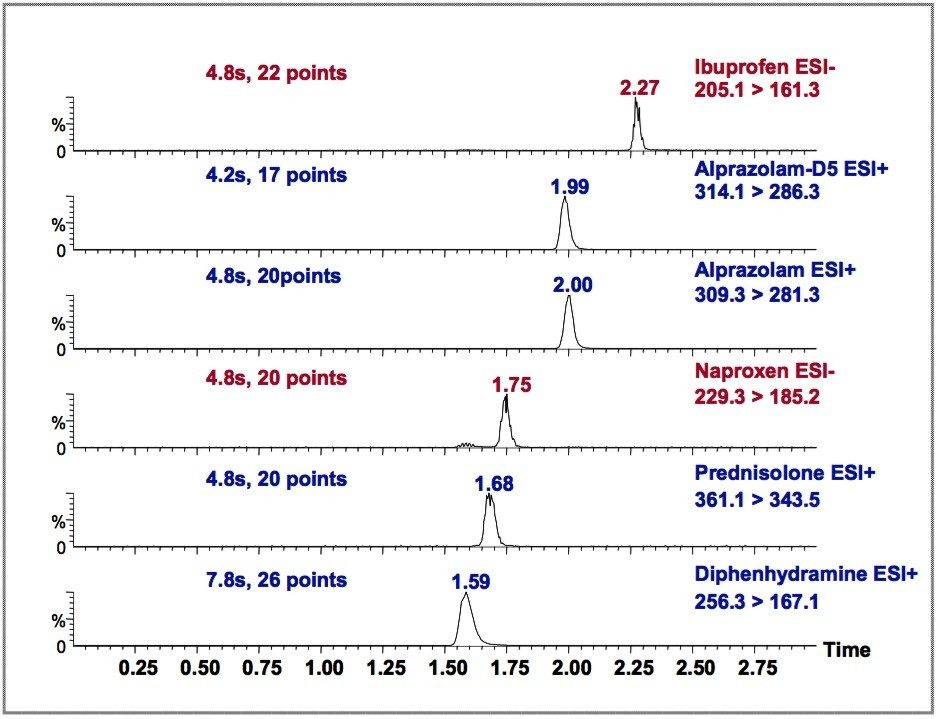
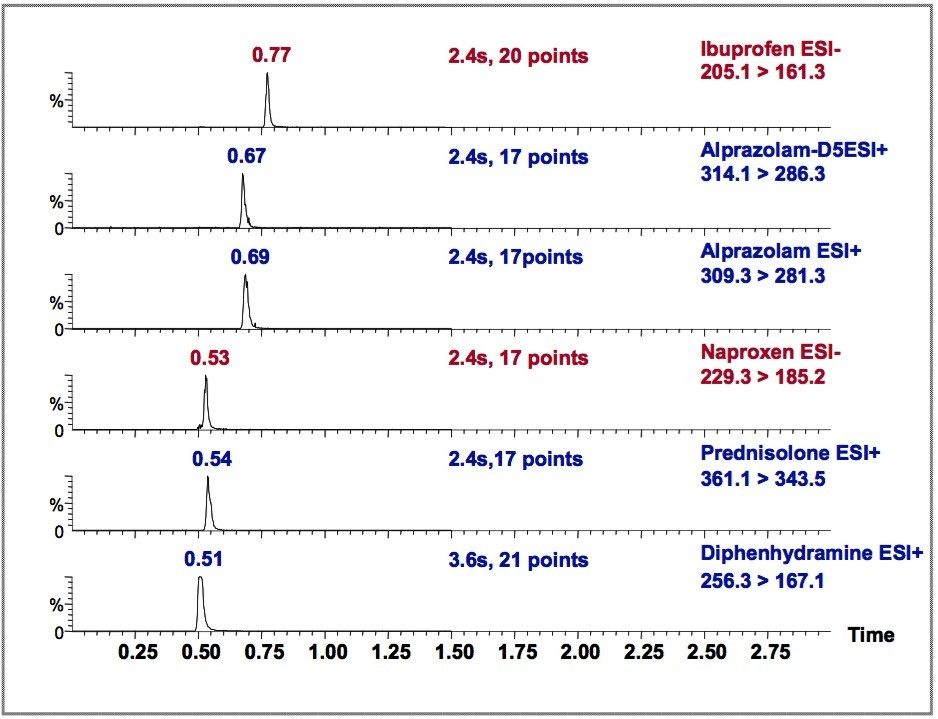
The LC conditions were kept as similar as possible for HPLC and UPLC (column dimension, injection volume, and mobile phase composition). The gradient conditions were individually optimized for HPLC and UPLC, such that a good separation was obtained from each. The flow rates entering the MS were kept constant for comparative purposes. In reality, for the UPLC separation, much higher flow rates could be implemented to further enhance throughput.
In order to demonstrate the effect of the MRM cycle time (especially the Dwell time) on the number of data points that can be collected per chromatographic peak, a second experiment was performed. The result is shown in Figure 4.
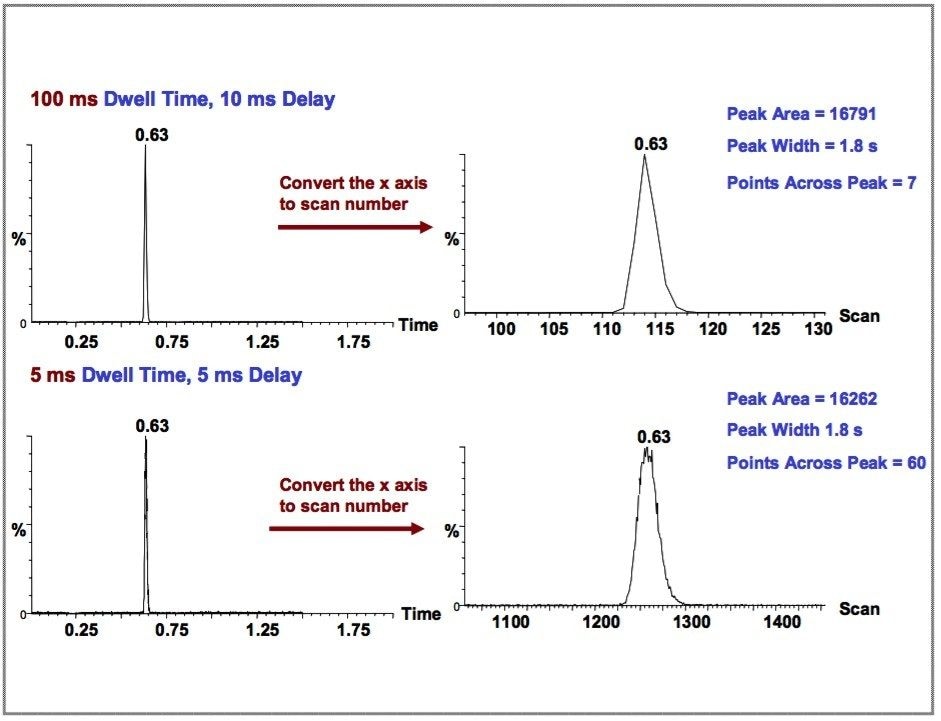
In this experiment, the same sample was injected twice on the same UPLC-MS/MS system. The chromatogram shown in Figure 4 (top) was collected with a dwell time of 100 ms and an interchannel delay of 10 ms, while the chromatogram shown in Figure 4 (bottom) was collected with a dwell time of 5 ms and an inter-channel delay of 5 ms. Seven data points were obtained in the top chromatogram and 60 data points were obtained in the bottom chromatogram. While the peak areas are similar for both, the integration precision is expected to be much better for the latter (with 60 data points collected across the peak).
When compared with the HPLC-MS/MS results, UPLC-MS/MS demonstrated faster separations, sharper peaks, and the sensitivity of the analysis significantly improved (Figure 5).
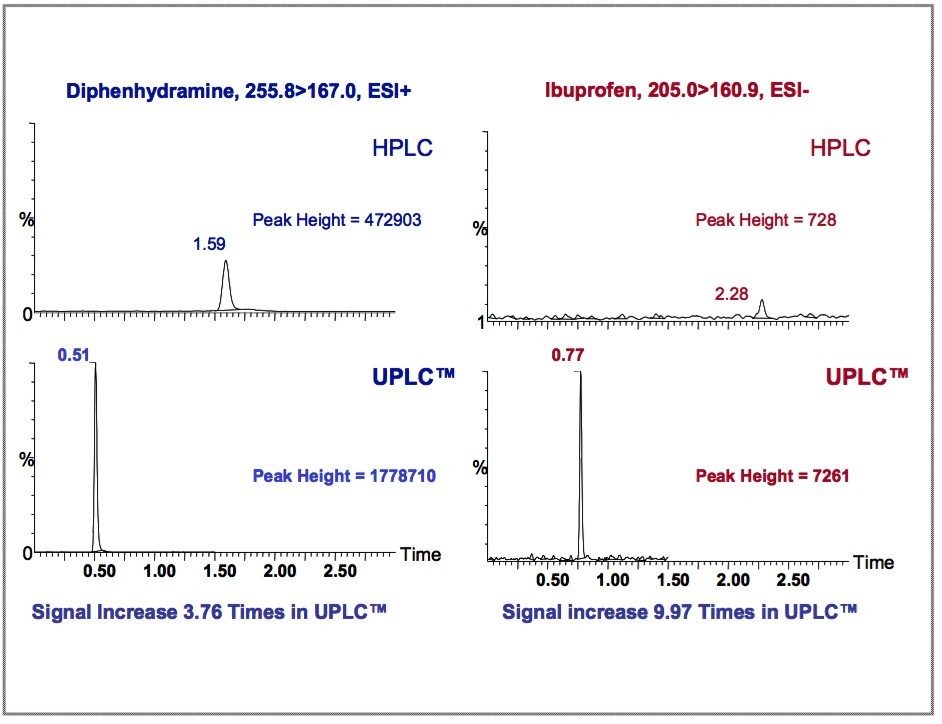
There are a few possible contributing factors for the signal increase in the UPLC-MS/MS data. The smaller particle size and system volume associated with the UPLC-MS/MS separation resulted in sharper peaks. Thus, for the same sample loading we obtained a more concentrated peak, with an increase in peak height. In addition, the extra resolution provided by UPLC reduced analyte co-elution, minimizing ion suppression.4
We have demonstrated the advantages of the ACQUITY UPLC System coupled with the Quattro Premier MS for high throughput LC-MS/MS separations. Compared with traditional HPLC, UPLC significantly enhances separation speed and sensitivity. The high data acquisition rate afforded by the Quattro Premier makes it an ideal complementary MS for detecting the extremely narrow LC peaks generated by the ACQUITY UPLC System.
720001120, February 2005