This application note describes an extraction and LC-MS/MS method for the quantification and confirmation of chloramphenicol in raw black tiger shrimp.
The global production of fish, as a food item, has greatly increased over the last few decades and is now at the highest levels on record. Currently, more than 15% of world protein consumption is provided by aquatic species, both captured and farmed. The growth in global human population means that the demand for fish will continue to rise, creating pressures on already over fished wild stocks of commercially caught species. Because of this demand, starting in the mid 1980s, there has been a steady increase in the quantity of fish produced by aquaculture, especially in Far Eastern and South American countries.1
Antibiotics are used in aquaculture, as in other farming methods, in order to prevent and treat bacterial diseases that would otherwise lead to increased animal mortality, reduced growth and lower yield at harvest. In the recent past there has been, in some countries, a tendency to overuse antibiotic treatments in order to compensate for deficiencies in aquacultural practice. The indiscriminate use of antibiotic compounds has led to concerns that it may cause increasing levels of drug resistance in organisms pathogenic to humans. Also, some of these compounds may have long term toxic effects when regularly consumed as residues in the diet.2

One of the most commercially lucrative species farmed in the Far East, and increasingly in South America, is the black tiger shrimp Penaeus monodon, Figure 1, which is exported in quantity to many Western nations.

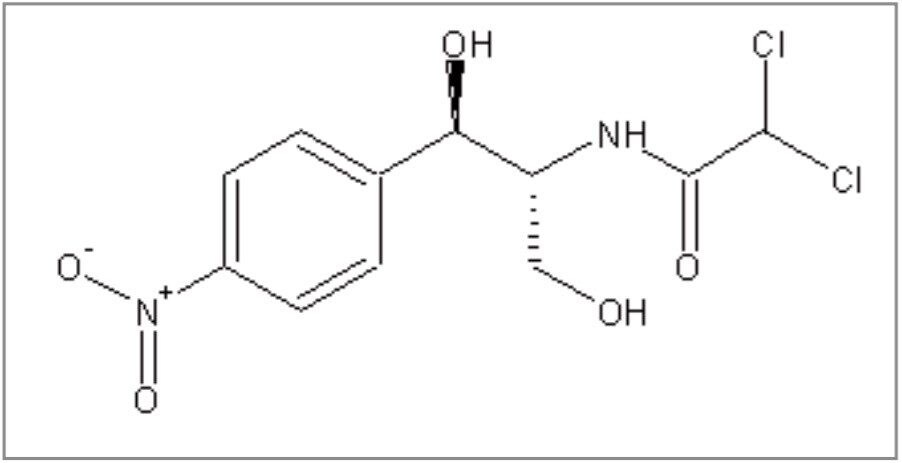
Recent shipments of shrimp, from various Far Eastern countries, into Europe, Canada and the United States of America, were found to be contaminated with residues of chloramphenicol Figure 2, a broadspectrum antibiotic used to treat bacterial infections in humans. Chloroamphenicol is a cause of a condition known as idiosyncratic aplastic anemia, a depression of the ability of bone marrow to produce blood cells. Approximately one in thirty thousand individuals have a hypersensitivity to chloramphenicol that may cause this aplasia irrespective of exposure level.3 For this reason, no Acceptable Daily Intake (ADI) can be determined and the drug has been listed in annex IV of the European Union Council regulation 2377/90/EEC. This means that it is prohibited for use in animals destined for human consumption and any produce contaminated with chloramphenicol residues may not be sold. Equivalent regulations apply in other Western countries.
There is no Maximum Residue Level (MRL) set for chloramphenicol in food of animal origin and any detection and confirmation of the compound will lead to condemnation of produce. For the protection of consumers it is necessary to have as sensitive an analytical method as possible in order to detect its unlawful use. Many thousands of metric tonnes of shrimp are exported to the West every year and a rapid, sensitive and confirmatory method is required to determine the presence and quantity of chloramphenicol in these exports. This paper describes an extraction and LC-MS/MS method for the quantification and confirmation of chloramphenicol in raw black tiger shrimp.
A quantity of peeled, de-veined, raw shrimp meat is finely chopped. 10 g shrimp meat is weighed into a 50 mL polypropylene test tube with screw cap lid and 2 mL water is added. Shrimp meat contains approximately 80% water. 10 mL acetone is added and the tube contents are macerated for 1 min using an Ultra Turrax homogeniser. The tube is then centrifuged at 6000 rpm for 5 mins. An 8 mL aliquot of the supernatant is transferred to a second polypropylene tube. 10 μL formic acid is added to the second tube and the contents mixed. 5 mL ethyl acetate is added and the tube is shaken for 1 min. The upper organic phase is transferred to a 14 mL capacity glass vial and the aqueous phase is re-extracted with 5 mL ethyl acetate. The extracts are combined, the vial is placed on a heated block at 40 °C and the solvent is evaporated under a stream of nitrogen. 1 mL aqueous 0.1% acetic acid is added to the vial together with 1 mL hexane. The vial is vortexed for 30 s and the hexane is carefully removed with a pipette. The aqueous sample is filtered through a 0.45 μm membrane filter into a 2 mL glass sample vial. The vials are spiked with deuterated internal standard prior to analysis. Using this method 1 mL analytical sample is equivalent to 4 g shrimp meat.
Chromatographic separation was carried out using an Waters Alliance System with the 2795 Separations Module and a 4.6 x 50 mm Symmetry C8 3.5 μm HPLC column. A 3.9 x 20 mm Symmetry C8 3.5 μm pre-column was placed upstream of the main analytical column. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. The sample injection volume was 50 μL and the mobile phase flow rate was 1 mL/min. The chromatographic gradient that was applied is shown in table 1.

Chloramphenicol was determined using a Waters Micromass Quattro micro API triple-quadrupole mass spectrometer with an electrospray ion source in negative polarity. Initial experiments were conducted to compare the sensitivity and selectivity of Single Ion Recording (SIR) MS with Multiple Reaction Monitoring (MRM) MS/MS acquisitions. Figure 3 shows chromatograms from SIR and MRM analyses of chloramphenicol spiked into blank shrimp matrix. The MRM method is highly selective for chloramphenicol, removing much of the baseline chemical noise and giving a much higher S:N value than that obtained using the SIR method. Therefore, an MRM method was chosen for the determination of chloramphenicol in this study.

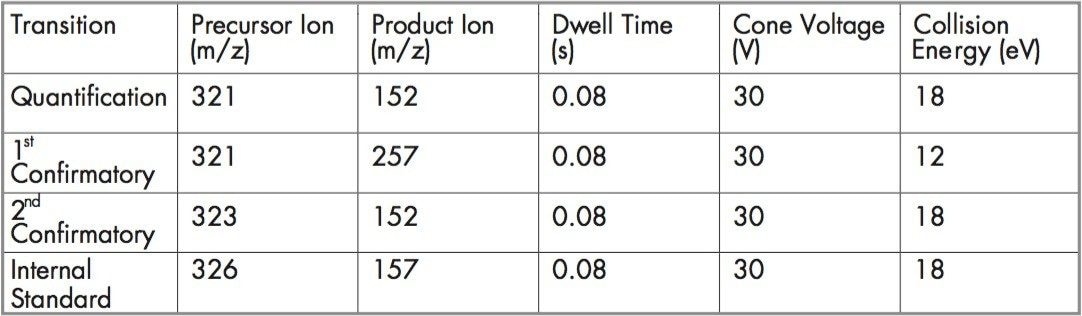
Table 2 shows the MRM transitions monitored, together with their dwell times, cone voltages and fragmentation collision energies.
Chromatograms corresponding to these 4 transitions are shown in Figure 4.
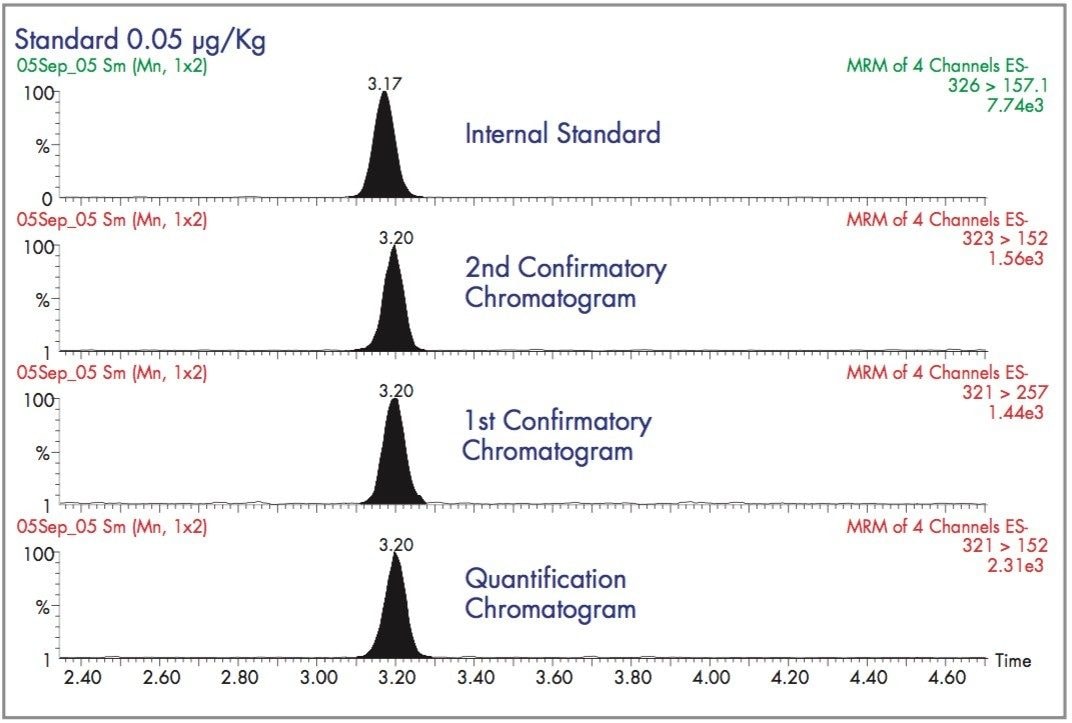
The abundance ratio of the quantification to the 1st confirmatory transition is 1.06. The abundance ratio of the quantification to the 2nd confirmatory transition is 1.53. The presence of chloramphenicol is considered confirmed if the observed abundance ratios do not deviate by more than 20% from these expected values.4
A series of matrix-matched calibration standards, spiked matrix samples, matrix blanks and recovery samples were analysed in order to determine method accuracy, linearity, precision, repeatability and recovery. The limit of determination (LoD) was also estimated. Internal standard was spiked at 0.5 μg/Kg in all samples. Calibration standards were made up at 0.025, 0.5, 0.2, 0.4, 0.6, and 0.8 μg/Kg. Spiked matrix samples were made up at 0.1 and 0.5 μg/Kg. Recovery samples were spiked at 0.1 μg/Kg prior to extraction.
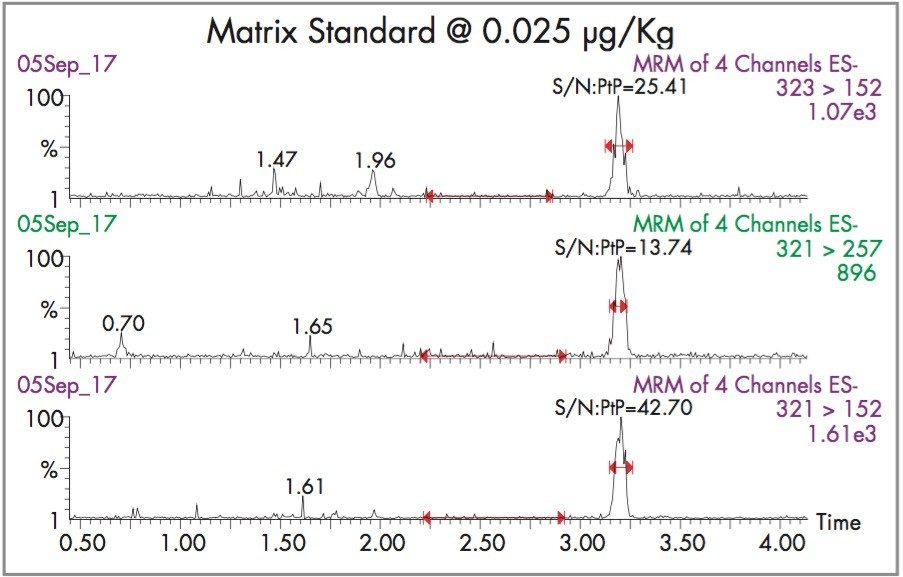
Figure 5 shows chromatograms for the three MRM transitions, taken from analysis of the calibration standard at 0.025 μg/Kg. The observed peak to peak S:N ratio for the most sensitive transition (m/z 321 > m/z 152) is 42:1. By extrapolation, the LoD, defined as the concentration at which S:N is 3:1, is approximately 0.002 μg/Kg.
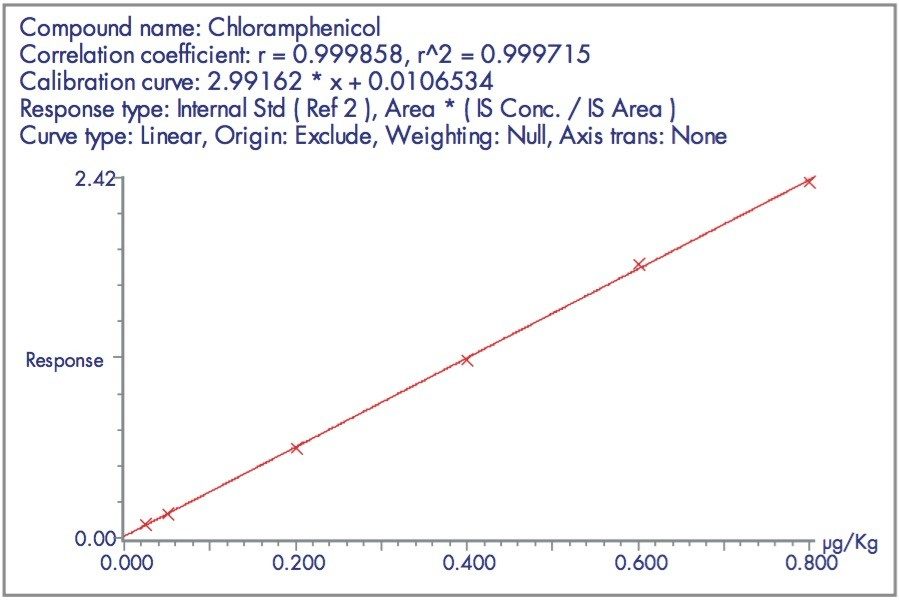
The linearity of the method is demonstrated by the calibration graph shown in Figure 6.
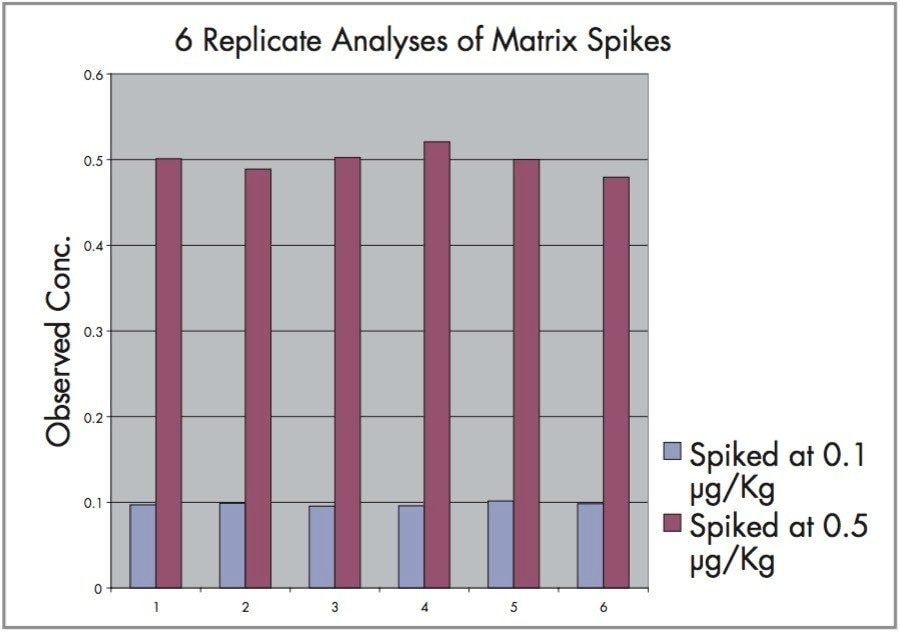
Method accuracy and precision are demonstrated by the graph in Figure 7. Six replicate analyses were performed on matrix spiked at 0.1 and 0.5 μg/Kg. Mean average observed concentrations were 0.10 and 0.50 μg/Kg and relative standard deviation values were 2.3% and 2.8% respectively.
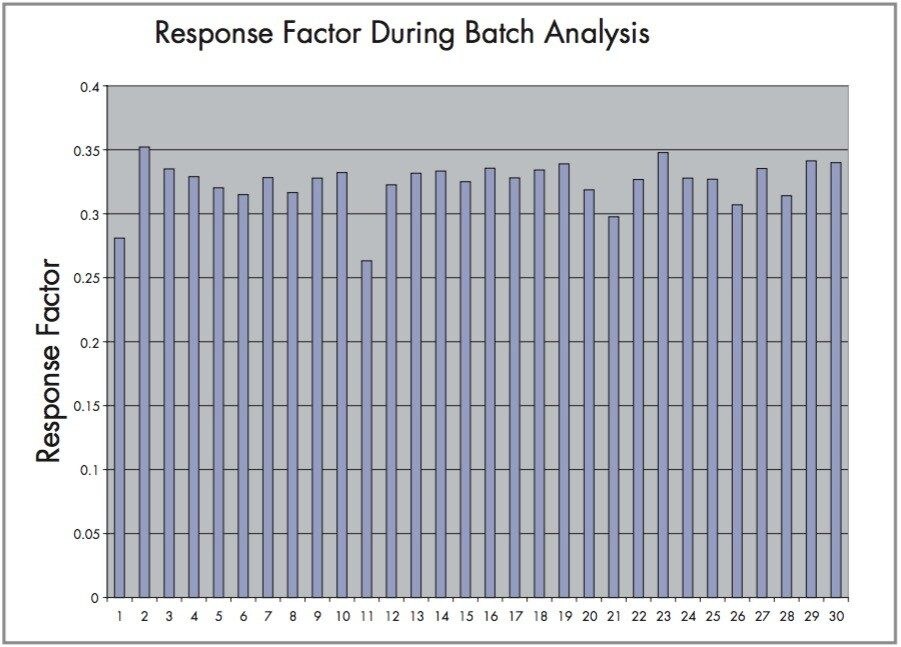
Method repeatability is shown by the graph in Figure 8. The response factor is shown for all standards and spiked samples during a batch analysis. The relative standard deviation of response factor is 5.7%.
Four recovery experiments were performed on shrimp spiked at the 0.1 μg/Kg level. Each sample was analysed three times, giving 12 determinations in total. The mean observed concentration was 0.092 μg/Kg with a relative standard deviation of 8.9%.
During a batch analysis of over 100 injections the ion abundance ratios of the transitions did not deviate by more than 20% from the expected values, even for the lowest calibrated level of 0.025 μg/Kg. This means that the method is able to confirm the presence of chloramphenicol at the lowest calibrated level. During this batch analysis the chromatographic peak retention time, width at half height and asymmetry factor remained constant.
A rapid and sensitive analytical method is required in order to protect consumers from shrimp contaminated with chloramphenicol. Multiple MRM transitions allow confirmatory criteria to be met whilst providing excellent sensitivity and selectivity. A quick extraction and short sample analysis time mean that this method may be applicable in a high throughput environment.
720000767, November 2003