A dual electrospray ion source interfaced to an orthogonal acceleration-time-of-flight mass spectrometer (oa-Tof-MS) is described. The novel design has been used to analyse a six component synthetic polymer additive mixture by liquid chromatography-mass spectrometry for exact mass measurement confirmation. The interface provides a simpler, more robust method of obtaining exact mass measurements through infusion of a reference compound into a separate electrospray inlet rather than post column addition presently used with a single electrospray probe. The results show negligible or no inter-channel 'cross talk' and exact mass measurements of <5 ppm.
Polymer additives have several key functions. They aid processing and finally characterize the formulated commercial product within the field of polymer chemistry. Typical additives act, for example, as light stabilizers, antioxidants, flame retardants or plasticizers.
Polymer additive analysis is important because of possible health and environmental risks that can arise from the manufacture of certain plastic materials, which are intended to come into contact, for example, with foodstuffs.1
The possibility of migration of the additives into foodstuffs needs to be carefully monitored and therefore the levels of additives in the plastic need to be determined and well regulated.
Often added at levels between 0.1–3% w/w, the determination of the fate of these additives during and after use, their presence in competitor products and inherent purity presents a daunting analytical challenge. Therefore, precise identification is a crucial requirement in their characterization. Synthetic polymer formulations are complex, therefore extraction procedures from the polymer matrix usually precede chromatographic separation.
Electrospray mass spectrometry and tandem mass spectrometry have been previously employed on some of the additives studied.2 Here high and low collision energies were used to produce nominal mass product ion spectra. From the results mechanistic and fragmentation pathways were proposed.
For this study, oa-Tof-MS has been conveniently used with liquid chromatography for the exact mass measurement of the same group of additives previously investigated.2
Exact mass assignment by liquid chromatography oa-Tof-MS is presently achieved through post-column addition of a suitable reference material into the solvent flow prior to entry into the ionization region of the mass spectrometer. The reference material is added solely to be used as a single point 'lock mass' against which any subsequently acquired mass spectra are mass measured.
However, post-column addition of a suitable reference into the solvent flow does have some practical limitations that have to be manually addressed. These include reference signal intensity fluctuations with liquid chromatography gradients, the possibility of mass interference with analyte material having the same nominal mass and ion suppression of the reference compound with high concentrations of analyte response. These practical limitations have been overcome by the design of a novel dual electrospray ionization source that samples both the analyte and reference streams independently.
The ion source was interfaced to an orthogonal-acceleration time-of-flight mass spectrometer (oa-Tof-MS) and the design and functionality of the source is described here. Evaluation of the dual spray source for exact mass measurement was performed by LC-MS on a six component synthetic polymer additive mixture.
Together with the novelty of the design, exact mass measurement of synthetic polymer additives does not appear to be cited in the literature and therefore have been investigated for a variety of such compounds of masses <1500 Da.
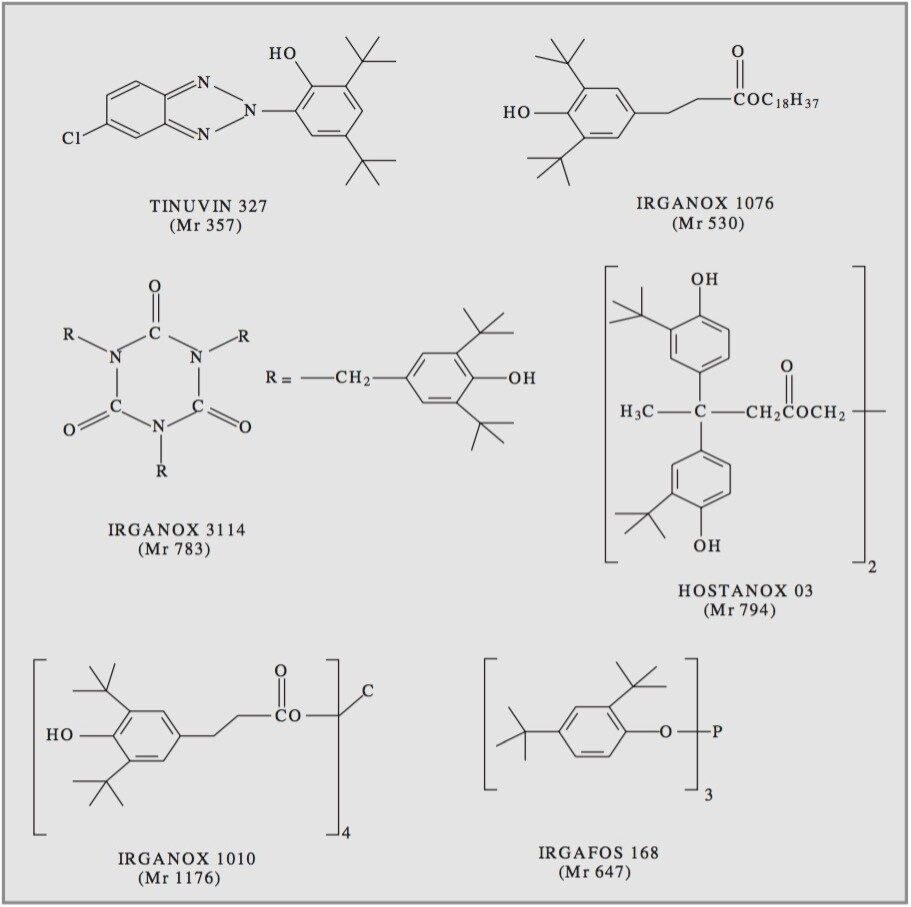
Pure synthetic polymer additives were used. The six polymer additives investigated consisted of antioxidants and light stabilizers. Their structures and relative molecular masses are shown in Figure 1. The additives were obtained from ICI (Wilton, UK) and used as received. 1 mg/mL stock solutions of each additive were prepared in (1:4, dichloromethane:methanol). A solution containing the additive mixture was prepared to a concentration of 10 ng/μL of each component. All solvents used were HPLC grade (Sigma-Aldrich, Poole, UK) and used without further purification.
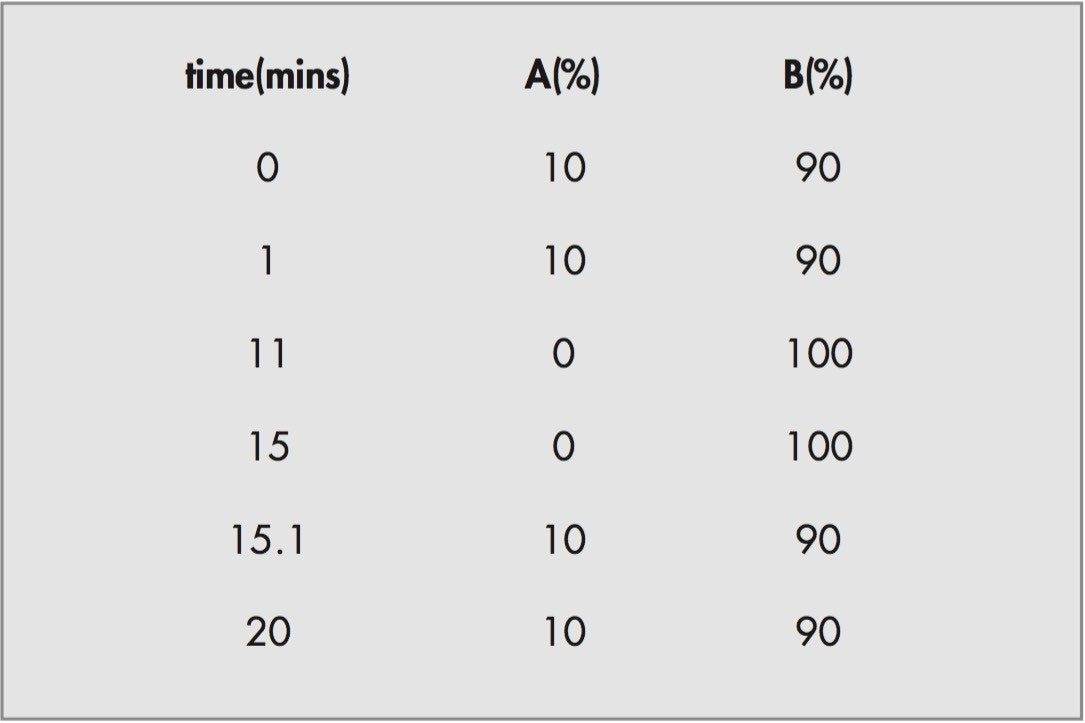
A Waters 2790 Alliance HT System (Waters Corp., Milford, MA, USA) provided the liquid flow at a rate of 300 μL/min to the mass spectrometer. The liquid chromatographic separation was performed on a Waters Symmetry C8, 2.1 x 100 mm, 3.5 μm Column. A 10 μL injection of the solution containing the additive mixture was injected on-column. The following gradient profile, as shown in Table 1, was used for the separation:
Mobile phase A: H2O + 0.1% HCOOH
Mobile phase B: CH3OH + 0.1% HCOOH
After elution the liquid stream exiting the column was introduced into the analyte channel of the dual electrospray source. See Figure 2.
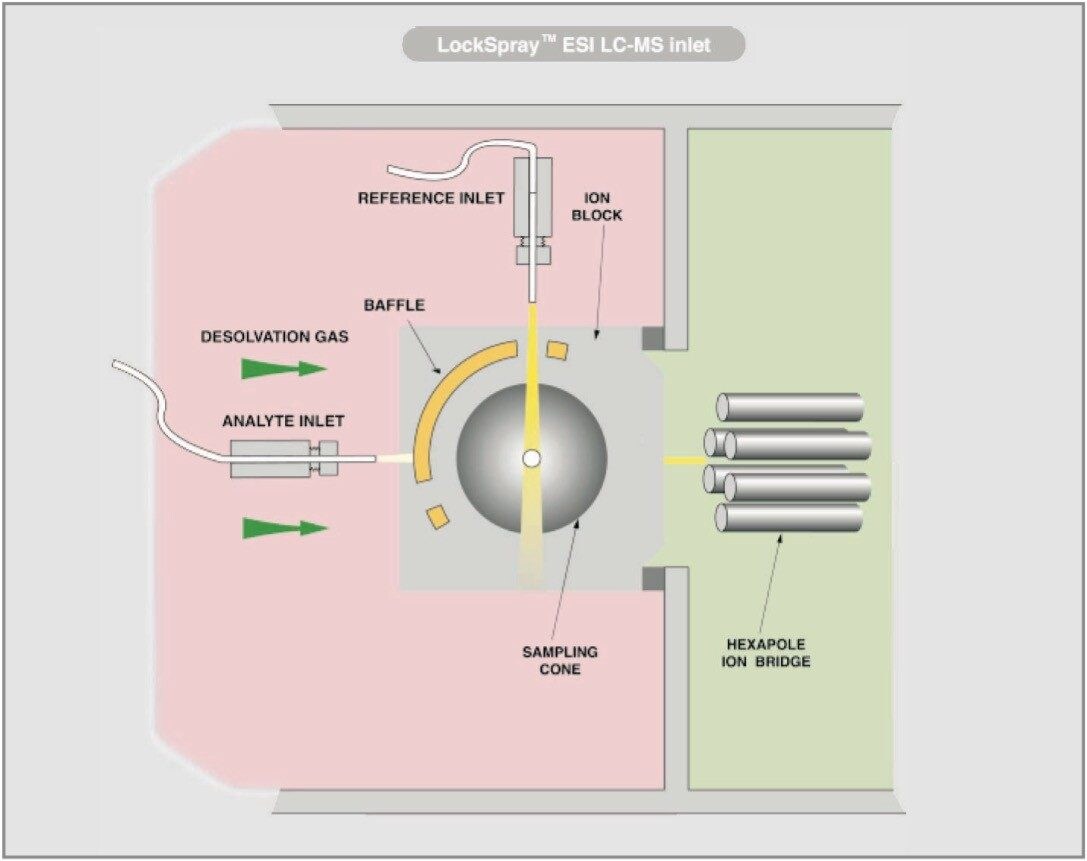
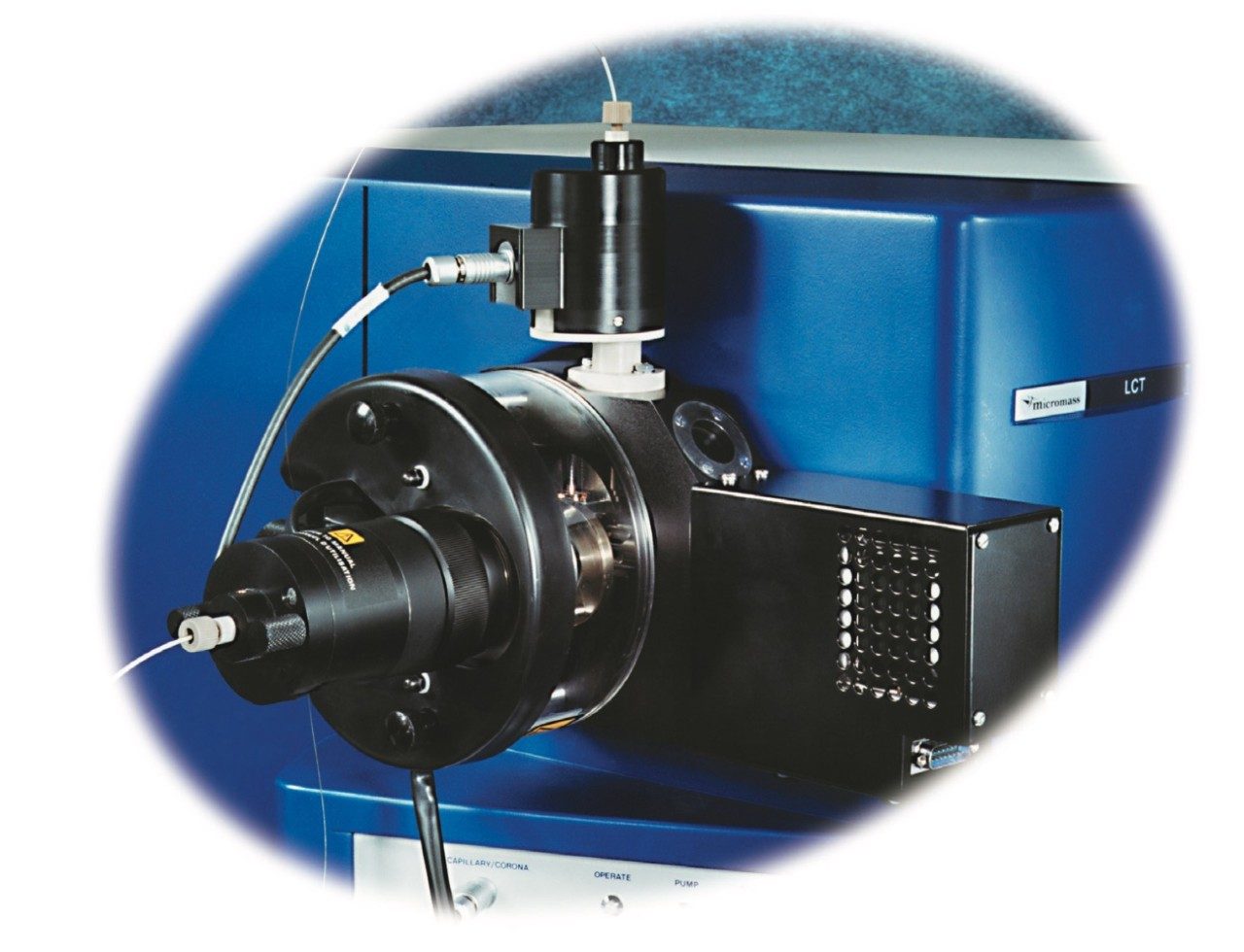
Mass spectra were acquired on a LCT oa-Tof Mass Spectrometer (Micromass UK Ltd, Manchester) which was fitted with a novel two way electrospray ionization source (LockSpray), see Figure 2. The dual source housing fits directly around the Z SPRAY source of the LCT with the reference sprayer positioned perpendicular to the analyte sprayer. No desolvation gas is required for the reference sprayer since low flow rates, typically <10 μL/min, are used. The same ionization voltage is applied to both the reference and analyte spray tips with an equal flow of nebulization gas assisting the liquid flow of each spray.
The spray from each capillary tip is sampled independently through use of a rotating baffle, which is externally driven by a stepper motor. The sprays are admitted to the sampling cone region of the LCT through an aperture in the rotating baffle. The two positions of the sampling rotor are indexed which allows data from each of the two sprays to be stored into separate fuctions within a single data file during an acquisition.
During the investigation the reference spray was sampled every five seconds. Mass spectra were collected in positive electrospray mode and acquired from 200–1250 Da at an acquisition rate of 1 spectrum/s with an inter-acquisition delay of 0.1s. The electrospray voltage was 3kV and cone voltages of 34V and 20V were applied to the analyte and reference materials respectively during an acquisition.
Exact mass measurement was provided by infusing leucine enkephalin ([M+H]+= 556.2771) into the reference channel of the dual source. This 'lock mass' was infused at a flow rate of 5 μL/min using a Harvard Apparatus (South Natick, MA,USA) Model 22 Syringe Pump at a concentration of 0.5 ng/μL (1:1, acetonitrile:H2O).
Three replicate LC-MS acquisitions were made to obtain the data. Data acquisition and processing was carried out using MassLynx (v3.5).
During an acquisition, ions from the two sprays are sampled independently and are stored in separate functions in a single data file. Figure 3 shows the total ion chromatograms obtained from an acquisition. The bottom trace was obtained from the analyte channel and the top trace from the reference channel.
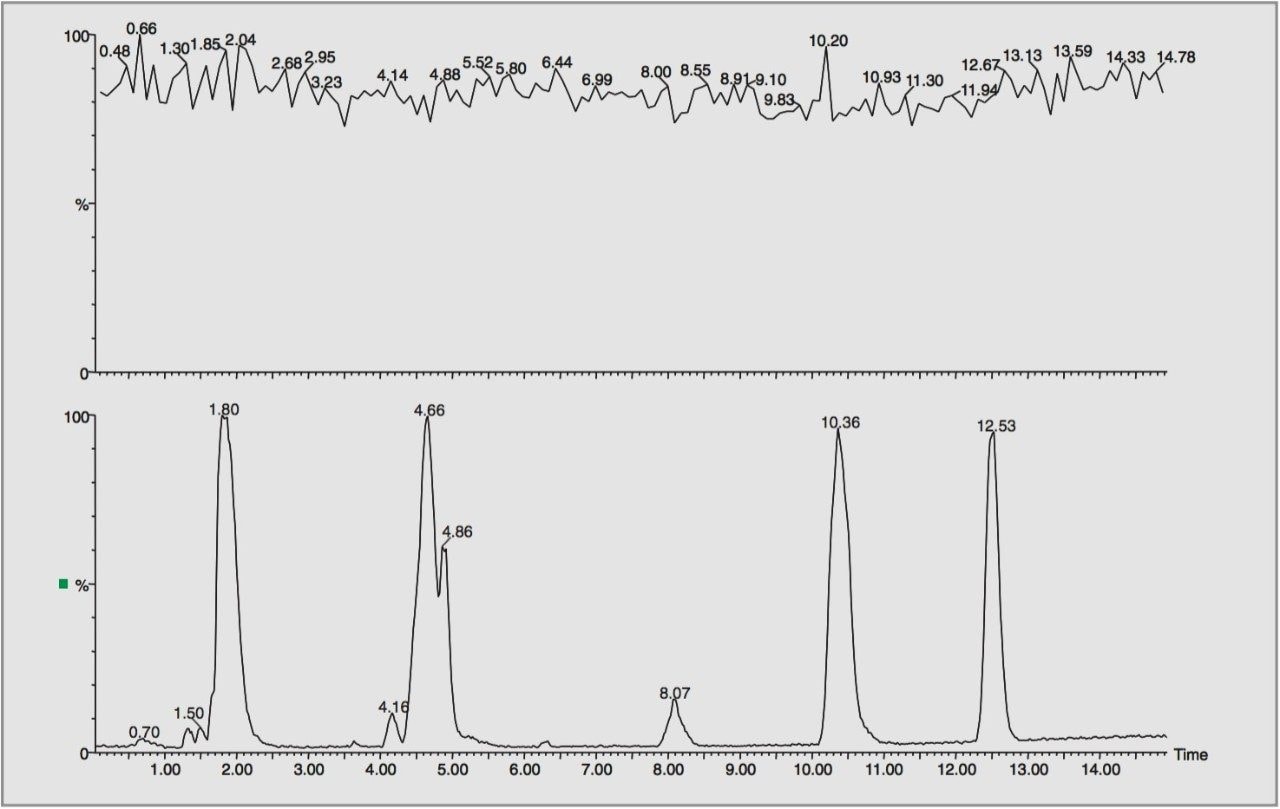
Figure 4 shows spectra obtained from the analyte (bottom trace) and reference (top trace) data files during the elution of the peak at 1.82 minutes. These spectra clearly show that cross-talk between the two channels is negligible. Fragment ions observed at m/z 645 and m/z 495 are consistent with previous investigations.
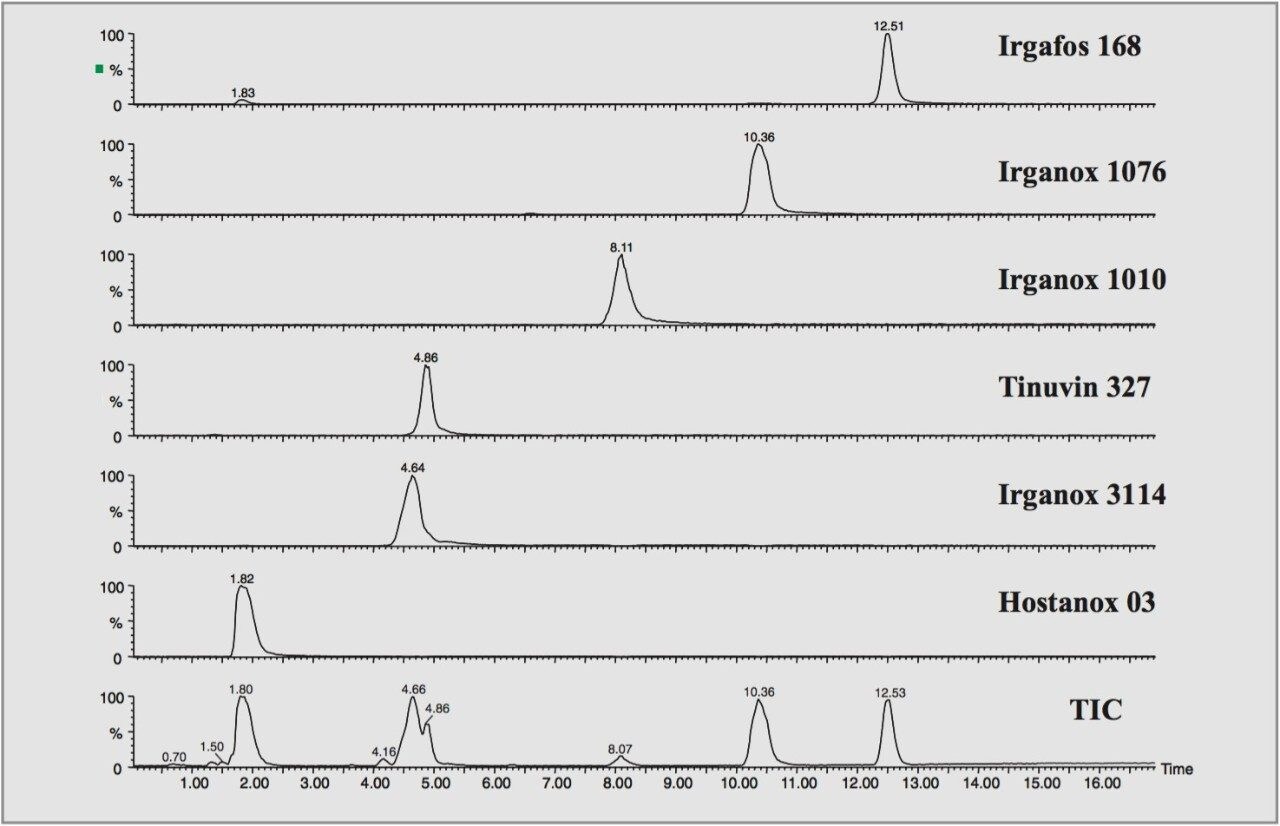
The mass chromatograms obtained from the separation of the six component mixture are shown in Figure 5. All of the additives are well resolved with the exception of Irganox 3114 and Tinuvin 327 where there is some degree of coelution. This however posed no problem for exact mass measurement since they are well resolved by their individual masses.
Typical background subtracted mass spectra achieved are shown in Figure 6. Fragment ions observed with Irganox 1076 from the protonated molecule are consistent with previous investigations.2
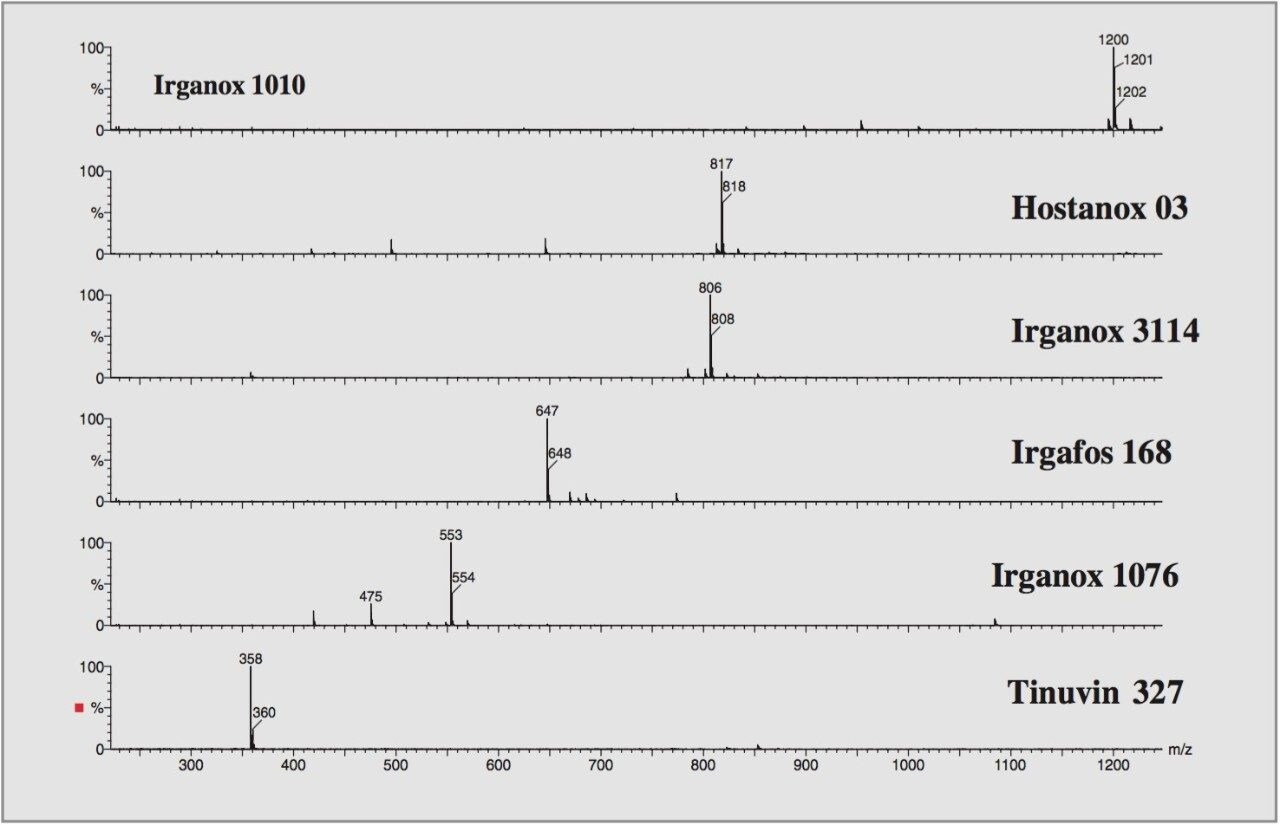
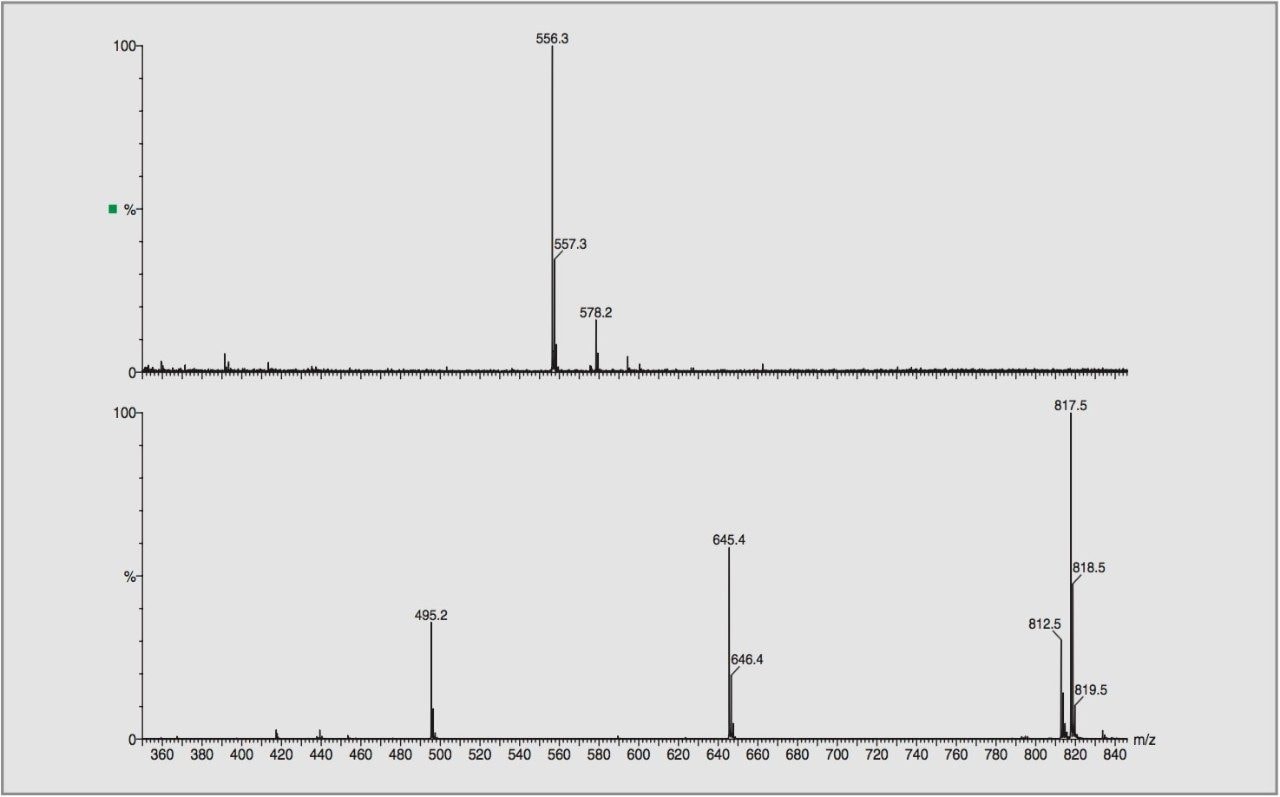
Figure 7 shows an example of a 'lock mass' corrected exact mass spectrum achieved for Irgafos 168.
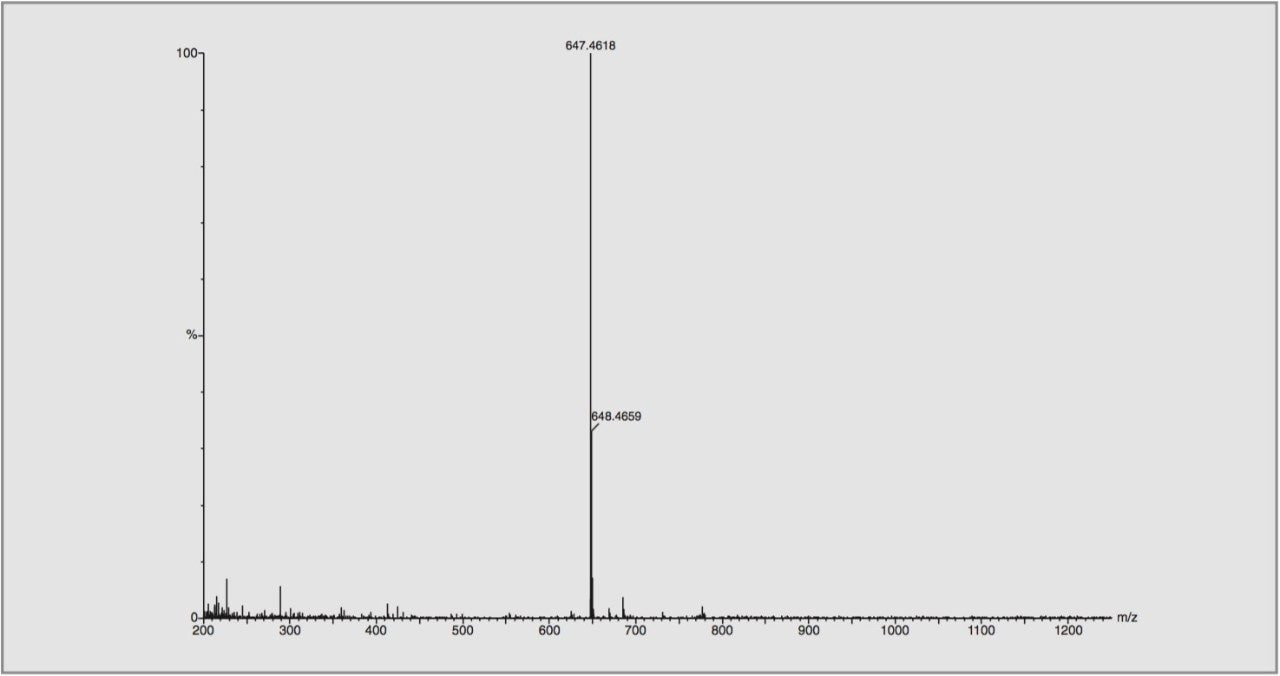
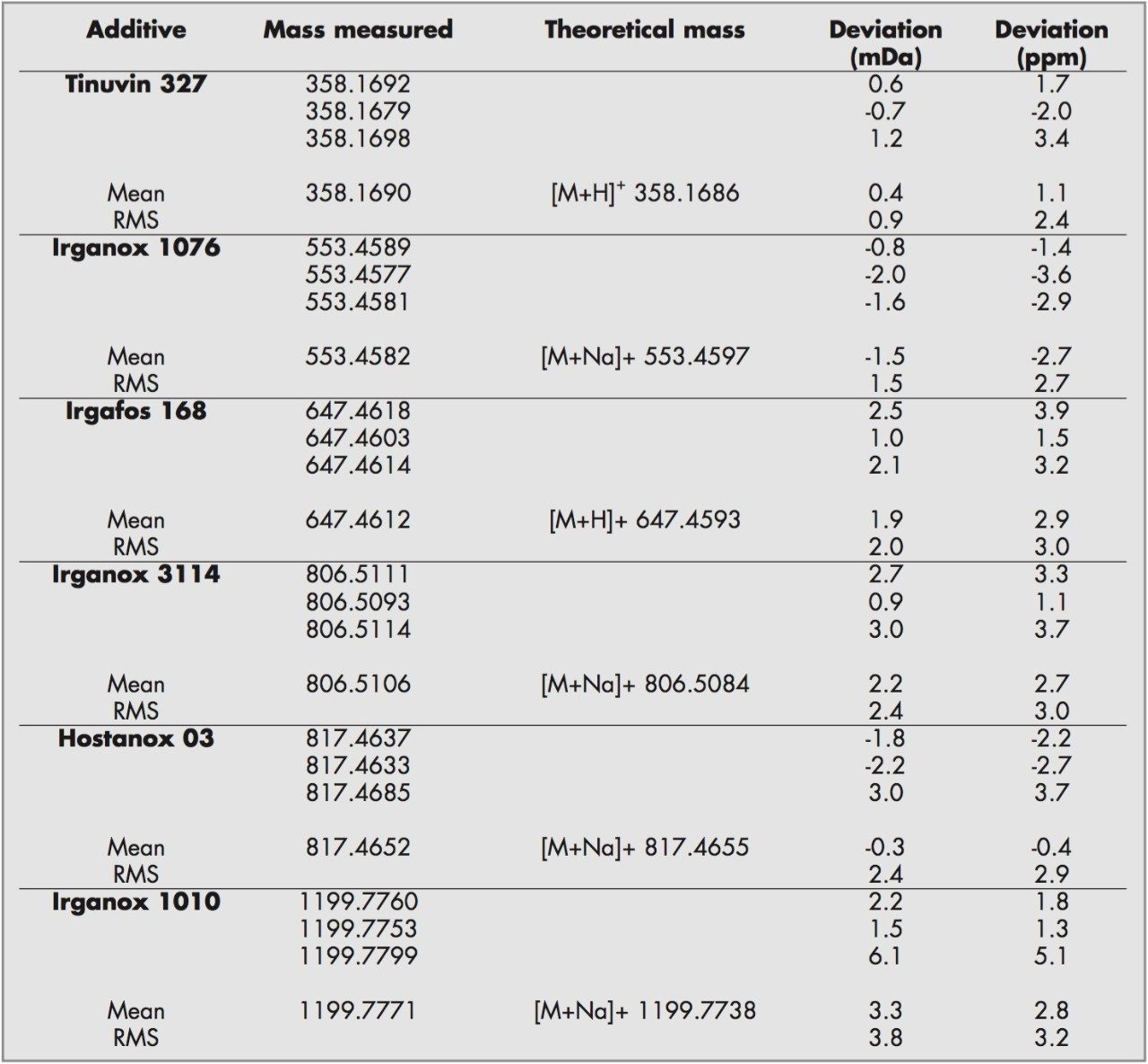
For exact mass measurement of each additive, five spectra from the reference data file were combined to produce an averaged spectrum at the same point in time as the analyte data to be mass measured. The exact mass data obtained are shown in Table 2. The results gave mass errors <5 ppm from the theoretical mass, which would allow sufficient accuracy for assignment of an elemental formula.
The dual electrospray interface has been successfully interfaced to the LCT. The exact mass measurement results for six synthetic polymer additives have been obtained with errors <5 ppm, therefore allowing confirmation of empirical formulae. The design allows exact mass measurements to be made more simply and robustly than with post-column addition of the reference compound, principally through elimination of suppression effects, possible mass interference and LC gradient effects which are sometimes inherent with post column addition.
720000535, October 2002