Ultra-Sensitive Quantification of Tirzepatide in Human Plasma Using Xevo TQ Absolute Mass Spectrometry with waters_connect Quantitation Software
Tirumal Datar, Dr.Padmakar Wagh
Waters Corporation, United States
Published on October 30, 2025
Abstract
An ultra-sensitive, specific, and robust liquid chromatography–tandem mass spectrometry (LC–MS/MS) method was developed and validated for the quantitative determination of tirzepatide in human plasma. The analysis was conducted using a Xevo™ TQ Absolute Tandem Quadrupole Mass Spectrometer equipped with an electrospray ionization (ESI) source operating in positive ion mode. The sample preparation involved protein precipitation followed by solid-phase extraction (SPE). Chromatographic separation was performed on an ACQUITY™ Premier UPLC™ H-Class Plus System using reversed-phase C18 column under gradient elution conditions. To ensure precise data acquisition and streamline workflow processes, the waters_connect™ for Quantitation Software was used. The method exhibited excellent peak resolution, retention time consistency, recovery, matrix effects, and overall reproducibility. The linear dynamic range was determined to be 0.125 to 55 ng/mL, with a lower limit of quantification (LLOQ) of 0.25 ng/mL, demonstrating exceptional sensitivity. Inter- and intra-day precision and accuracy were within acceptable limits as per bioanalytical method guidelines (M10 Bioanalytical Method Validation and Study Sample Analysis FDA-2019-D-1469). Tirzepatide remained stable under various storage and handling conditions. This validated method offers a powerful analytical tool for the reliable quantification of tirzepatide in human plasma, supporting its application in pharmacokinetic, bioavailability, and clinical research studies.5,7,8
Benefits
- Advanced quantification platform: Xevo TQ Absolute Mass Spectrometer enables ultra-sensitive, robust, and selective quantification of tirzepatide for clinical and research applications.
- Efficient sample preparation via SPE: SPE ensures consistent recovery, enhanced analyte enrichment, and superior matrix cleanup compared to protein precipitation or liquid-liquid extraction (LLE).
- Minimized matrix effects: SPE effectively removes plasma interferences, reducing matrix effects and improving accuracy at sub-nanogram concentrations.
- High sensitivity and dynamic range: Achieve an LLOQ of 0.250 ng/mL with excellent signal-to-noise and broad dynamic range for pharmacokinetic applications.
- Optimized for peptide analysis: The method supports accurate quantification of low-abundance peptide drugs like tirzepatide in complex biological matrices.
Introduction
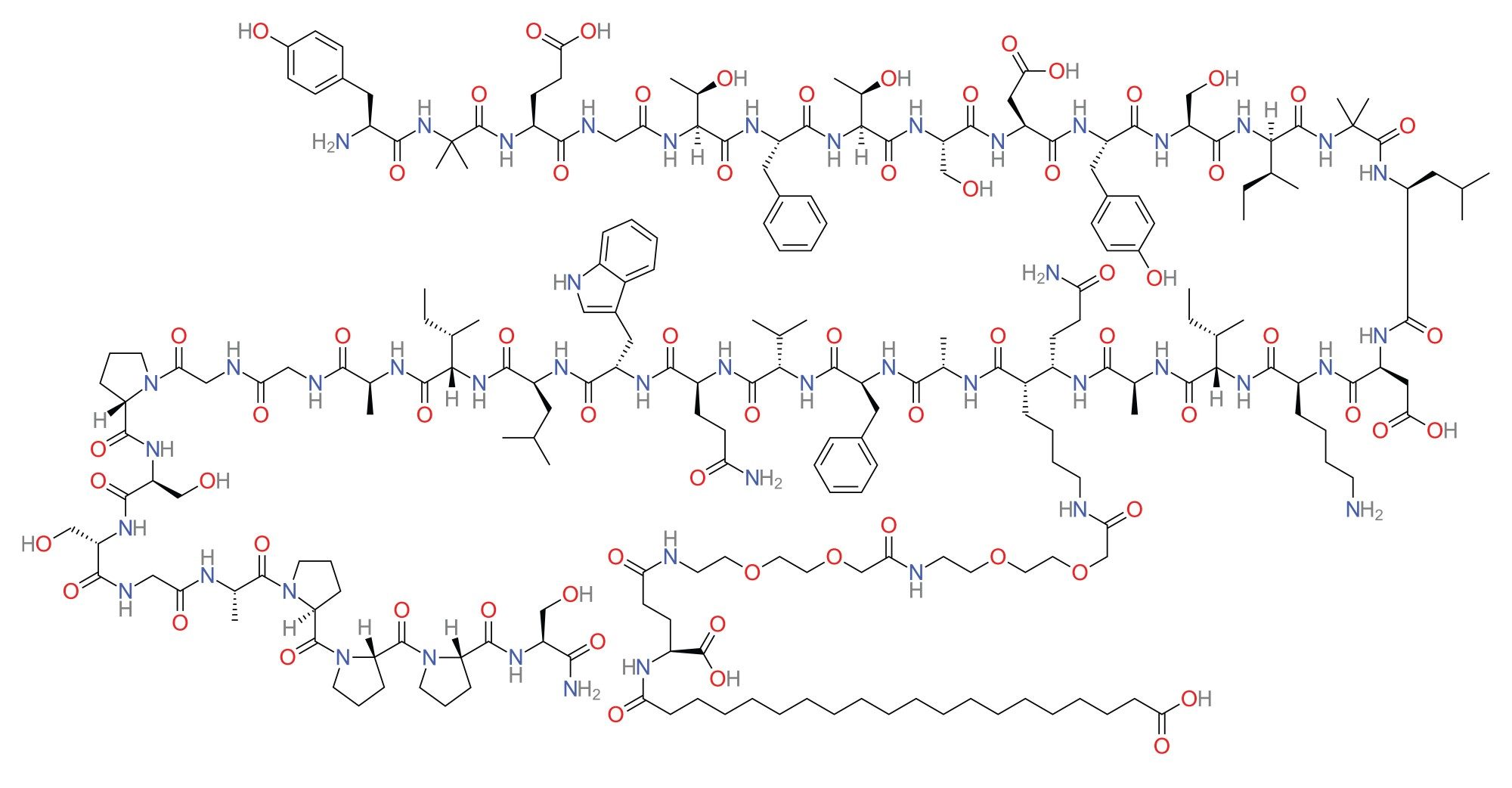
Tirzepatide is a novel, long-acting dual incretin receptor agonist that targets both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. As a synthetic peptide, it demonstrates potent antihyperglycemic and weight-reducing effects, making it a promising therapeutic candidate for the treatment of type 2 diabetes mellitus (T2DM) and obesity.
Structurally designed for enhanced receptor affinity and metabolic stability, tirzepatide combines the efficacy of dual receptor activation with a favourable pharmacokinetic (PK) profile, allowing for once-weekly subcutaneous administration. Preclinical and clinical studies have consistently demonstrated tirzepatide’s superior effects on glycemic control, insulin sensitivity, and body weight reduction compared to traditional GLP-1 receptor agonists.
Tirzepatide exhibits a complex pharmacokinetic profile, characterized by a long half-life of approximately five days, supporting its weekly dosing regimen. Plasma concentrations vary considerably based on dosage and formulation, with peak levels reaching several hundred ng/mL and declining gradually over time. Therefore, the development of a highly sensitive and reliable bioanalytical assay is critical to support PK and pharmacodynamic (PD) studies. Such an assay must be capable of accurately quantifying tirzepatide across a wide concentration range from ultra-trace levels during the elimination phase to high concentrations following peak exposure. This is essential for dose optimization, therapeutic monitoring, and throughout the drug development lifecycle.
Experimental
Mass Spectrometry
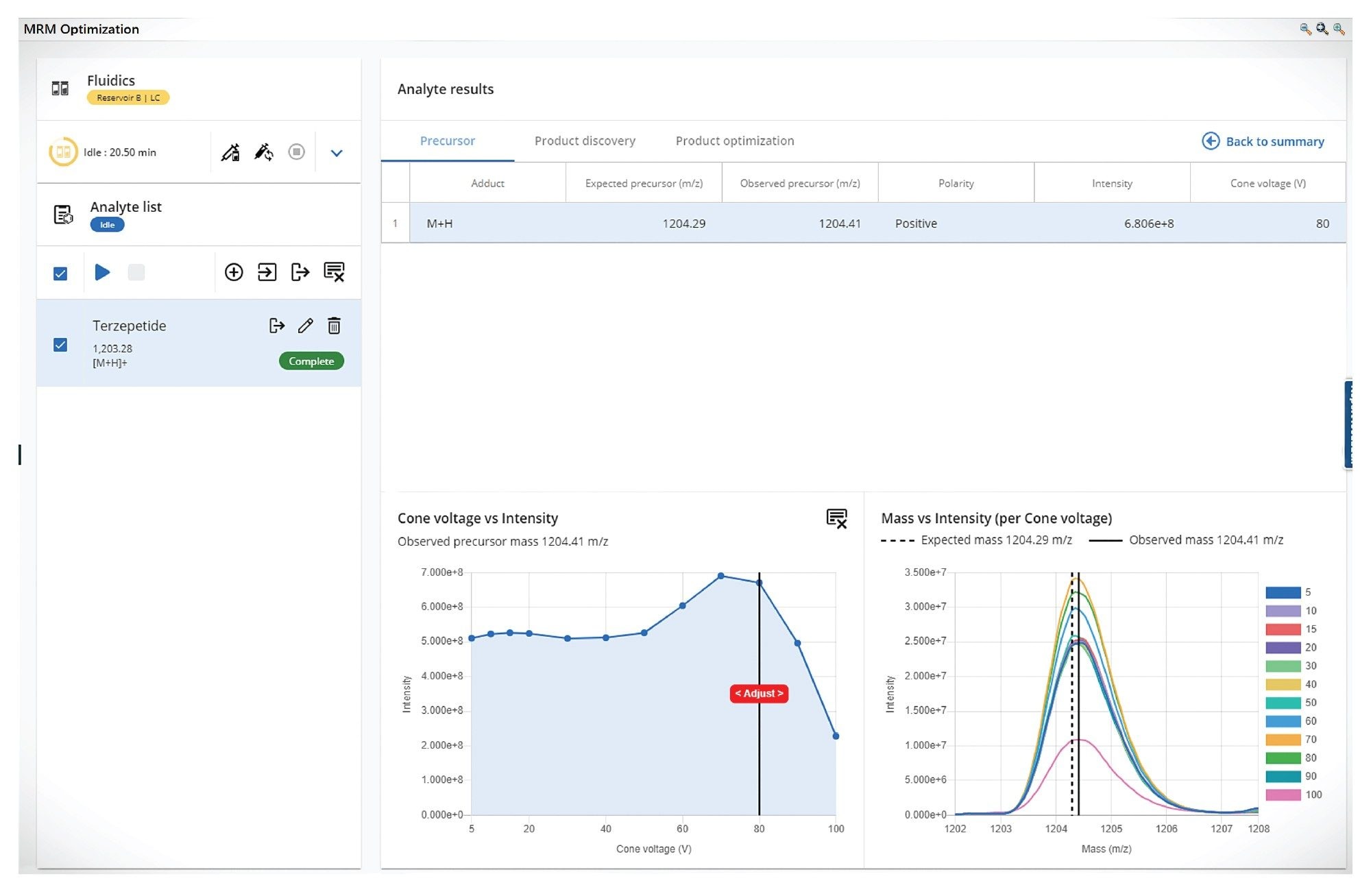
The MS MRM conditions were optimized using the waters_connect for Quantitation optimization tool. This offers an integrated and automated environment to streamline the tuning process for automated precursor ion detection. The software identifies the most intense charge states during direct infusion. Product ion scanning collision energy ramps are used to generate a spectrum of fragment ions, aiding in the selection of the most stable and sensitive MRM transitions. Key instrument parameters, including cone voltage, collision energy, source/desolvation temperature, and capillary voltage were systematically optimized to achieve maximum sensitivity and reproducibility as shown in the Figure 2.
Sample Preparation
For this study, standard and spiking solutions were diluted using water containing 2% formic acid. To create the calibration curve from 0.125 to 55.0 ng/mL, control human plasma was spiked with known quantities of tirzepatide standard. Additionally, quality control (QC) samples were prepared with tirzepatide spiked at four different levels: 0.25 ng/mL (LLQC), 1.25 ng/mL (LQC), 12.5 ng/mL (MQC), and 55.0 ng/mL (HQC). As no stable isotopically labeled internal standard was available, the assay was performed using external calibration.
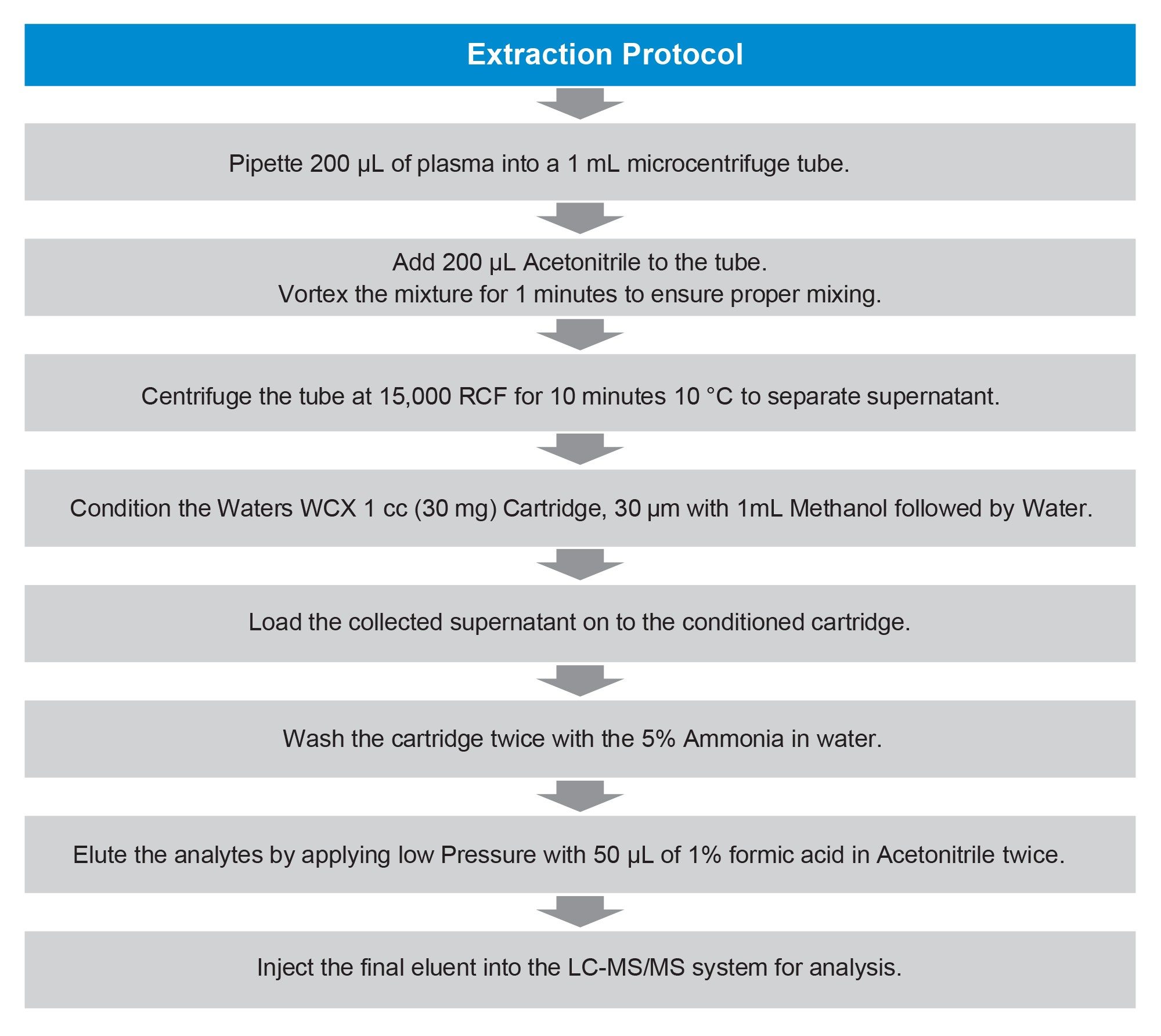
200 µL of human plasma was treated with an equal amount of acetonitrile. After thorough vortex mixing for one minute, the samples were centrifuged at 15000 RCF at 10 °C for 10 minutes. The resulting supernatant was then subjected to SPE using Waters™ WCX 1 cc (30 mg) Cartridge, 30 µm, as outlined in the SPE protocol described in Figure 3.
LC Conditions
|
LC system: |
ACQUITY H-Class PLUS UPLC System with FTN Sample Manager |
|
Vials: |
QuanRecovery™, MaxPeak™ 12 x 32 mm PP 300 µl Screw Cap Vials (p/n: 186009186) |
|
Column: |
ACQUITY Premier Peptide BEH C18, 300 Å, 1.7 µm, 2.1 x 100 mm) Column |
|
Column temperature: |
60 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
15 µL |
|
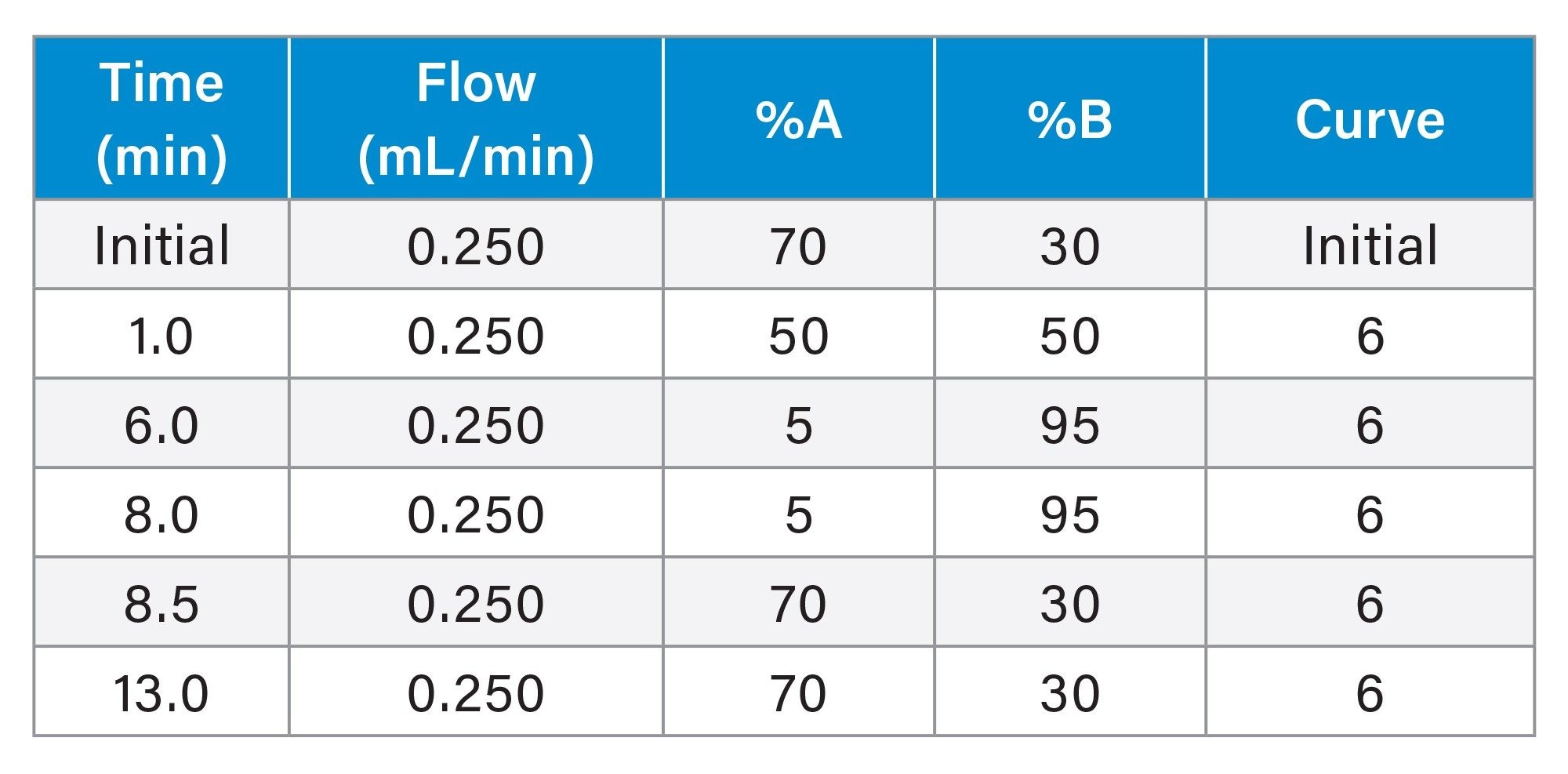
Mobile phase A: |
Water with 0.2% formic acid |
|
Mobile phase B: |
Acetonitrile with 0.2% formic acid |
Gradient Table
MS Conditions
|
MS system: |
Xevo TQ Absolute Mass Spectrometer |
|
Ionization mode: |
Electrospray (positive ion mode) |
|
Capillary voltage: |
3.50 kV |
|
Source temperature: |
150 °C |
|
Desolvation temperature: |
600 °C |
|
Desolvation gas flow: |
1100 L/hr |
|
Cone gas flow: |
300 L/hr |
|
Cone voltage: |
50 V |
MRM Method

The dwell times were set automatically using the auto dwell function to acquire a minimum of 10 data points across each peak.
Data Management
|
MS software: |
waters_connect for Quantitation Software |
Results and Discussion
In this study, a robust and optimized sample preparation protocol was established in conjunction with a UPLC–MS/MS analytical method for the accurate quantification of Tirzepatide in human plasma. Solid-phase extraction (SPE) was employed using a Waters WCX 1 cc (30 mg) mixed-mode cartridge, which combines weak cation exchange and hydrophobic interactions, offers enhanced selectivity for peptide-based analytes and enables efficient removal of matrix components, thereby improving assay robustness and sensitivity. The 30 µm particle size provided a larger surface area, contributing to improved extraction efficiency and method reproducibility. This comprehensive and reliable extraction approach, utilizing Waters mixed-mode solid-phase extraction (SPE) cartridges, provided a strong foundation for sensitive and reproducible chromatographic and mass spectrometric analysis. recovery. The use of the Waters WCX 1 cc (30 mg) mixed-mode cartridge provided enhanced selectivity for peptide-based analytes, enabling efficient removal of matrix components with minimal interference observed in blank plasma. The method demonstrated a consistent recovery of approximately 55%.
Instrument tuning played a pivotal role in ensuring the sensitive and accurate detection of Tirzepatide ions during mass spectrometric analysis. Optimal tuning parameters were essential to maximize the instrument’s response for the target analyte, thereby enabling reliable identification and quantification. Tirzepatide, like many peptide-based molecules, demonstrates multiple charging behaviour under electrospray ionization (ESI) conditions due to its structural composition. This behaviour is typical for large biomolecules, which contain several basic amino acid residues such as lysine and arginine that readily accept protons during ionization, as a result, the multiple charge state offer different precursor ions to investigate in order to obtain the most sensitive and selective MRM method. Tirzepatide forms multiple protonated species, as shown in Figure 4.
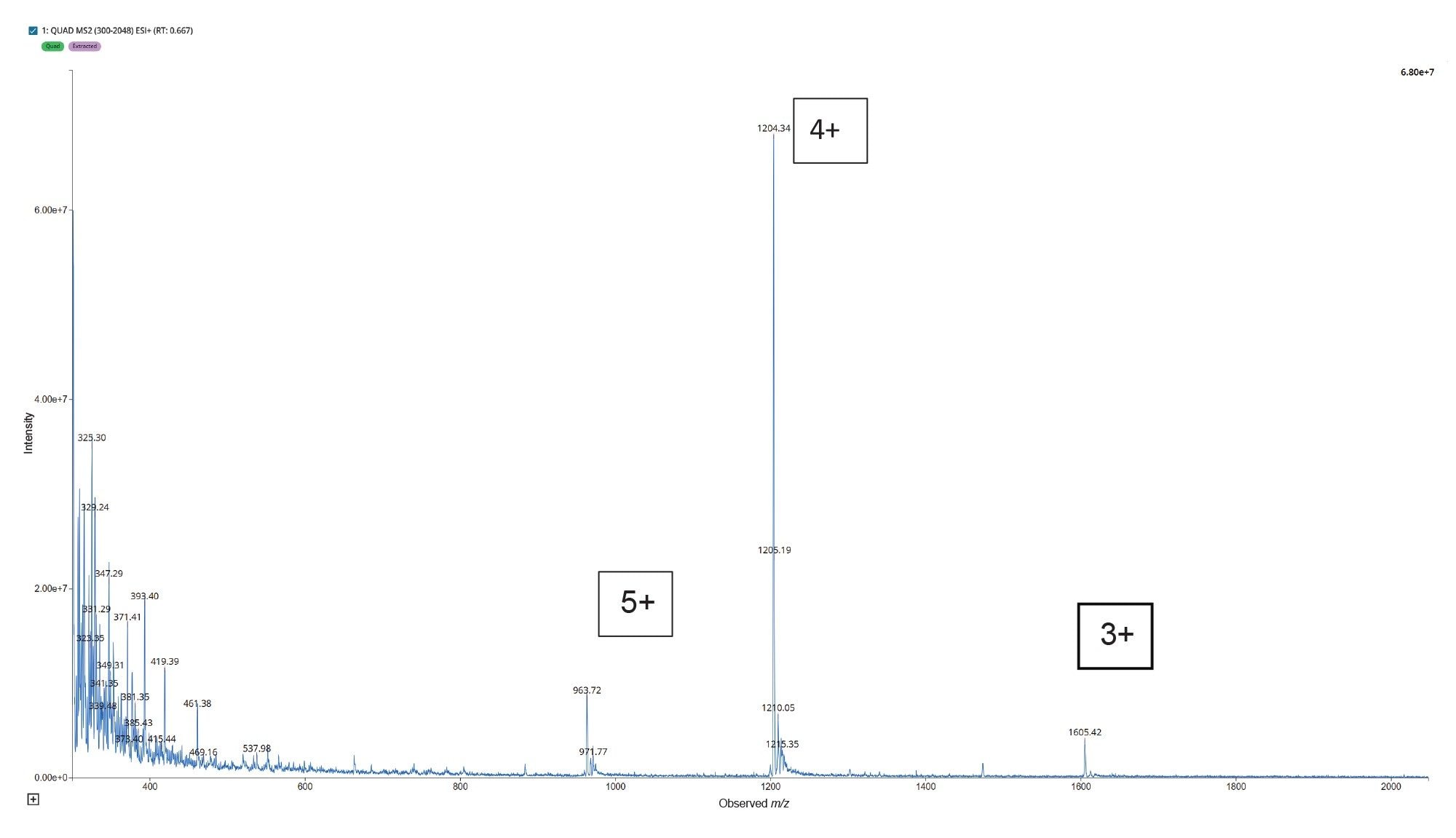
For optimal sensitivity, multiply charged precursors were evaluated, with the [M+4H]⁴⁺ precursor providing the best performance. The transitions monitored at m/z 1204.28>396.29 demonstrated the highest signal intensity and reproducibility, making them ideal for quantitative analysis.
Chromatographic separation was achieved by using ACQUITY Premier Peptide BEH C18, 300 Å, 1.7 µm, 2.1 x 100 mm) Column. ACQUITY Premier Columns are specifically designed to reduce nonspecific binding of compounds that interact with transition metals, thus providing reduced system condition, lower compound absorption, enhanced sensitivity and improved chromatographic peak shape. This column is designed specifically for peptide separations as it offers excellent retention and selectivity by providing hydrophobic interactions with the peptides. The column provided high resolution, sensitivity, and reproducibility for tirzepatide separation from matrix.
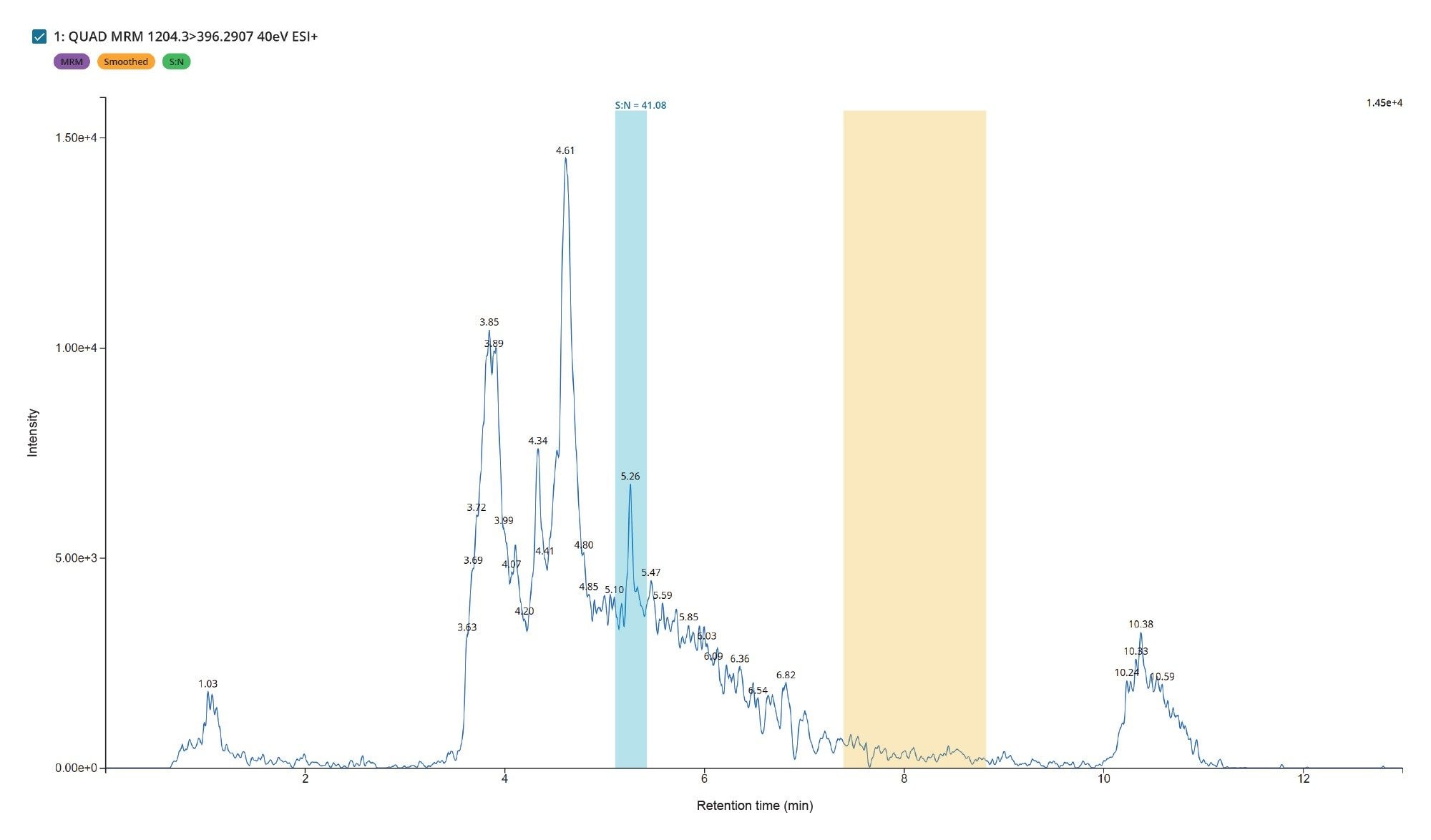
The developed method demonstrated excellent selectivity, sensitivity, and reproducibility. The drug peak eluted with a retention time of tR = 5.26 minutes and was well resolved from endogenous matrix peaks. Figure 5 shows a typical chromatogram of the matrix blank and a spiked sample at the LLOQ of 0.250 ng/mL, where the analyte appeared as an excellent symmetrical peak at 5.26 minutes, matrix effect was not observed. Additionally, the signal-to-noise (S/N) ratio at LLOQ (0.250ng/mL) exceeded 10 without smoothening, as shown in Figure 6.
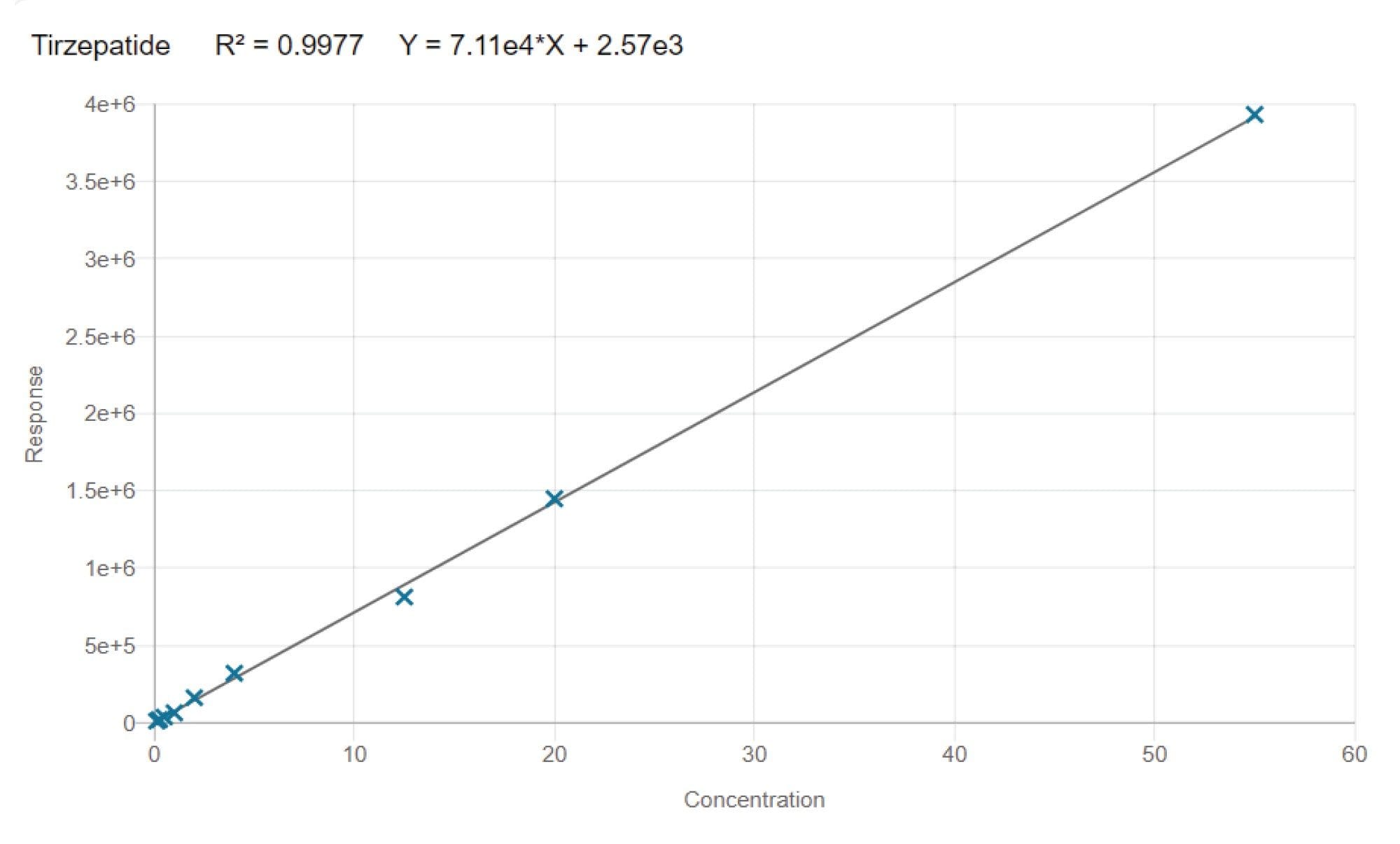
Linearity was assessed over the concentration range of 0.125 ng/mL to 55 ng/mL, yielding a correlation coefficient of 0.997 using 1/x weighting. A representative calibration curve within this range is depicted in Figure 7.
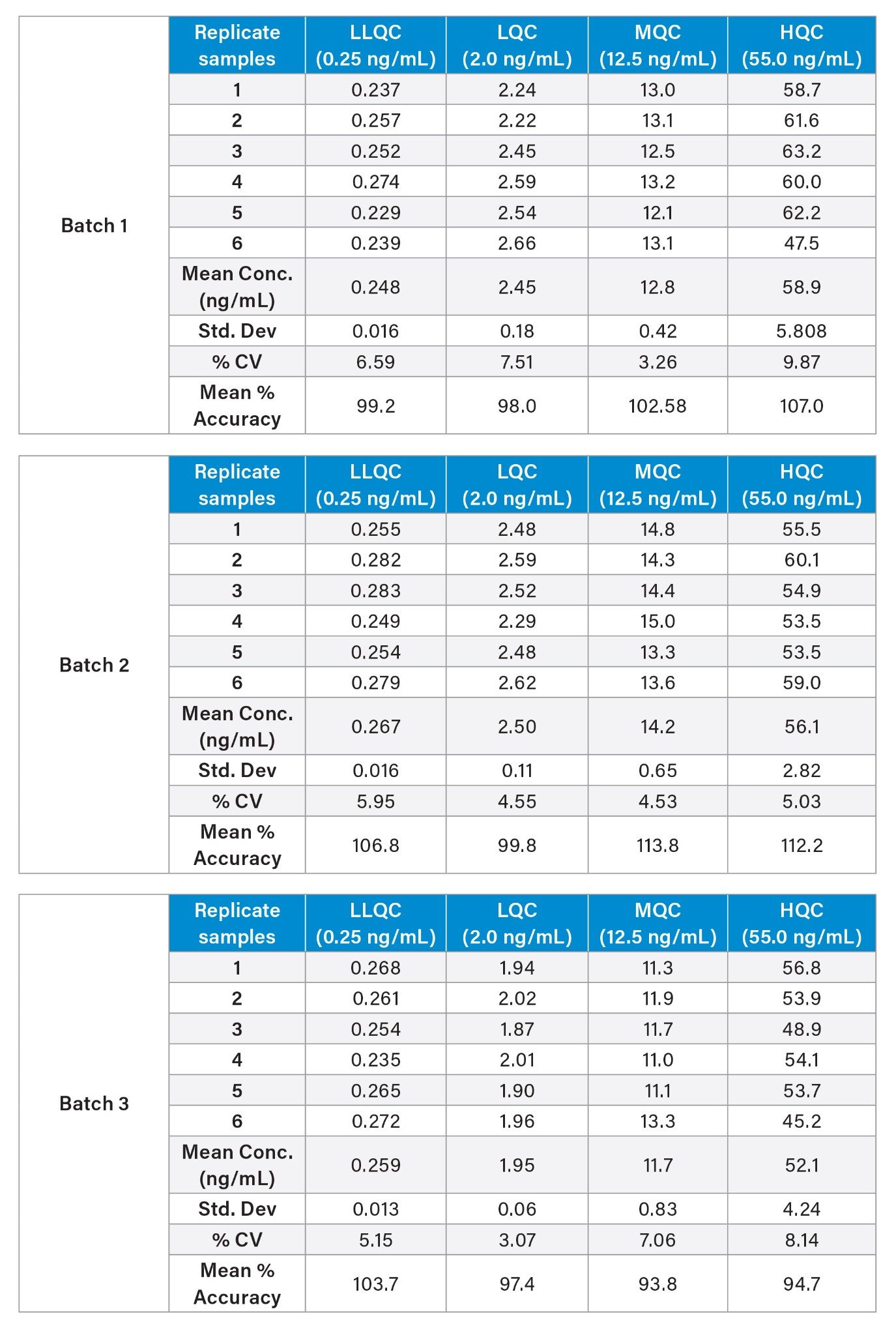
For precision and accuracy (P and A) evaluation, QC samples were spiked at four different levels: 0.25 ng/mL (LLQC), 2.00 ng/mL (LQC), 12.5 ng/mL (MQC), and 55.0 ng/mL (HQC). Three separate P and A batches were acquired on different occasions by different chemists. The results are summarized in Table 4.
The % accuracy in pre-spiked plasma samples at all QC levels fell within an acceptable range at respective QC levels. The coefficient of variation at each QC level in all three batches was below 10% RSD. Moreover, the mean % accuracy at the four QC levels in all three batches ranged from 92% to 115%, meeting the acceptance criteria outlined in the bioanalytical validation guidelines.
Conclusion
A robust and reliable UPLC–MS/MS method was successfully developed and validated for the accurate quantification of tirzepatide in human plasma. The sample preparation strategy, employing a Waters WCX 1 cc (30 mg) mixed-mode reversed-phase and ion-exchange sorbent, enabled efficient extraction with high reproducibility. The Xevo TQ Absolute Tandem Quadrupole Mass Spectrometer operating in ESI positive mode ensured highly sensitive and precise quantification, with optimized tuning parameters contributing to analytical performance.
Chromatographic separation on the ACQUITY Premier Peptide BEH C18 Column provided excellent retention, selectivity, and resolution, suitable for complex peptide analysis. Method validation results confirmed outstanding linearity, accuracy, and precision across the concentration range of 0.125–55.0 ng/mL, with all QC levels meeting regulatory acceptance criteria.
This validated method offers a powerful analytical tool for pharmacokinetic, clinical, and therapeutic monitoring of tirzepatide, supporting its continued development in diabetes research and contributing to advancements in bioanalytical methodologies for peptide therapeutics.
References
- Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos V. Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules. 2022 Jul 5;27(13):4315.
- Nauck MA, D’Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovascular diabetology. 2022 Dec;21(1):1-6.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A. Tirzepatide Once Weekly for the Treatment of Obesity. New England Journal of Medicine. 2022 Jun
- PubChem. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. bChem Compound Summary for CID 156588324, Tirzepatide; [cited 2022 Dec. 4] 4. Erni F. Use of high-performance liquid chromatography in the pharmaceutical industry. Journal of Chromatography A. 1990 May 16; 507:141-9.
- Nikolin B, Imamović B, Medanhodzić-Vuk S, Sober M. High performance liquid chromatography in pharmaceutical analyses. Bosn J Basic Med Sci. 2004 May;4(2):5-9.
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes mellitus: Insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, S.; Basit, A.; Chan, J.C.N.; et al. IDF Diabetes atlas: Global, regional and country-0.005.0010.0015.0020.0025.000.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0Conc. (ng/mlDays Recovery Plot of TirzepatideRat-1Rat-2Rat-3Rat-4Rat-5Rat-6).
- https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management.
720009073, October 2025