Application Brief
This is an Application Brief and does not contain a detailed Experimental section.
Jinxia Xia, Jo-Ann M. Jablonski, Darcy Shave, Kathy Lawrence
Waters Corporation, United States
Published on July 02, 2025
This is an Application Brief and does not contain a detailed Experimental section.
Temperature is a useful parameter for optimizing separations in both analytical and preparative chromatography. As the temperature rises, mobile phase viscosity and system pressure decrease, and the selectivity of the separation changes. Temperature also impacts retention times, compound resolution, sensitivity, and overall run time. The increased resolution can potentially allow one to load more sample onto the column. Precise control of the separation temperature is essential to achieving reproducible results.
Although temperature control is widely implemented in analytical HPLC to improve separations and ensure consistent and reliable outcomes, temperature is seldom controlled or used as a parameter for manipulating chromatography at the preparative scale for two reasons. First, high flow rate separations occur at the temperature of the incoming solvent and efficient heating of the mobile phase entering the column is challenging at these high flow rates. Second, large diameter columns cannot be effectively heated from the outside simply by using insulation, heated blankets or ovens. Ideally, analytical separations developed with temperature control should be scaled to prep at the same temperature to eliminate variability in the chromatography which can make the target identification and isolation ambiguous or lead to product loss.
When used with the Waters AutoPurification System, Timberline’s column and mobile phase heaters provide precise and consistent temperature control for preparative separations, allowing easier scale-up from UPLC/UHPLC to preparative methods. This integrated approach minimizes the need for method redevelopment, improves reproducibility, enhances hydrophobic compound solubility and boosts operational efficiency, which is particularly valuable in small molecule isolations and biological applications such as oligonucleotide and peptide purification. Temperature control also enhances flexibility in purification workflows, supporting green chemistry initiatives by enabling the substitution of acetonitrile with methanol. Elevated temperature reduces system backpressure, allowing the usage of sub-5 µm particles in larger-dimension preparative columns (e.g., 10 mm ID), which may increase loading capacity, minimize run-times, reduce solvent consumption, shorten dry-down times and lower waste disposal costs. This technical brief illustrates that comparable purification results can be achieved using methanol with sub-5 µm 10 mm ID columns, an approach that is both environmentally sustainable and economically viable. It also illustrates the precision and stability of temperature control across various flow rates and mobile phase compositions, showcasing the reliability of this solution.

Methanol usually results in longer retention time than acetonitrile in reversed-phase. Figure 2 illustrates that for both solvents, the compounds eluted earlier when the temperature was increased to 60 °C from ambient conditions.
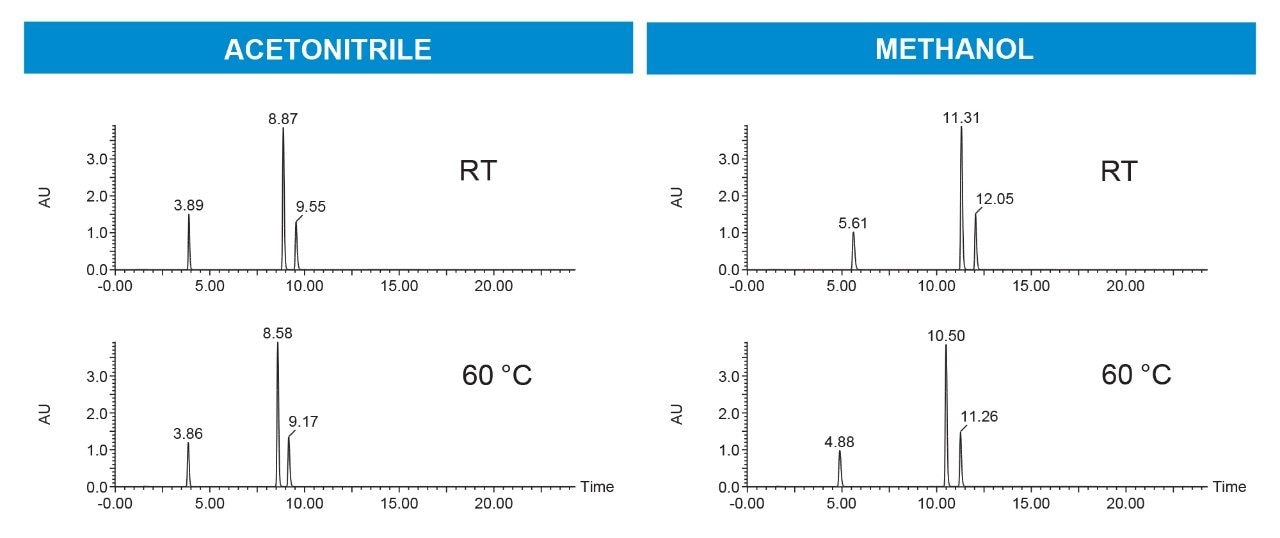
While temperature is a useful parameter for optimizing separations, it also reduces system and column backpressure, enabling the use of columns with smaller particle stationary phases. Small particle packings increase column efficiency and may enhance target compound peak areas and sensitivity. Reduced peak widths resulting from columns packed with small particles also produce smaller fraction volumes, ultimately decreasing fraction drying times. These factors all contribute to increasing overall operational efficiency and lowering processing costs. Figure 3 illustrates scaling the separation from UHPLC to the preparative scale using a 3.5 µm, 10 mm ID prep column. Figure 4 shows the separation on the 19 x 50 mm prep column at 24 mL/min at 60 °C.
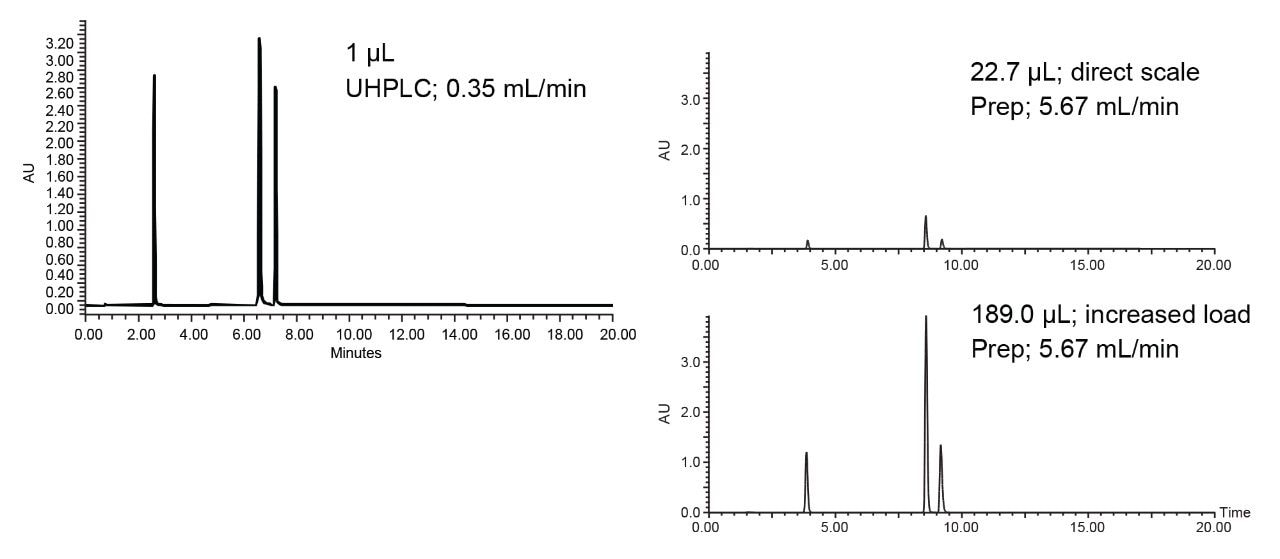
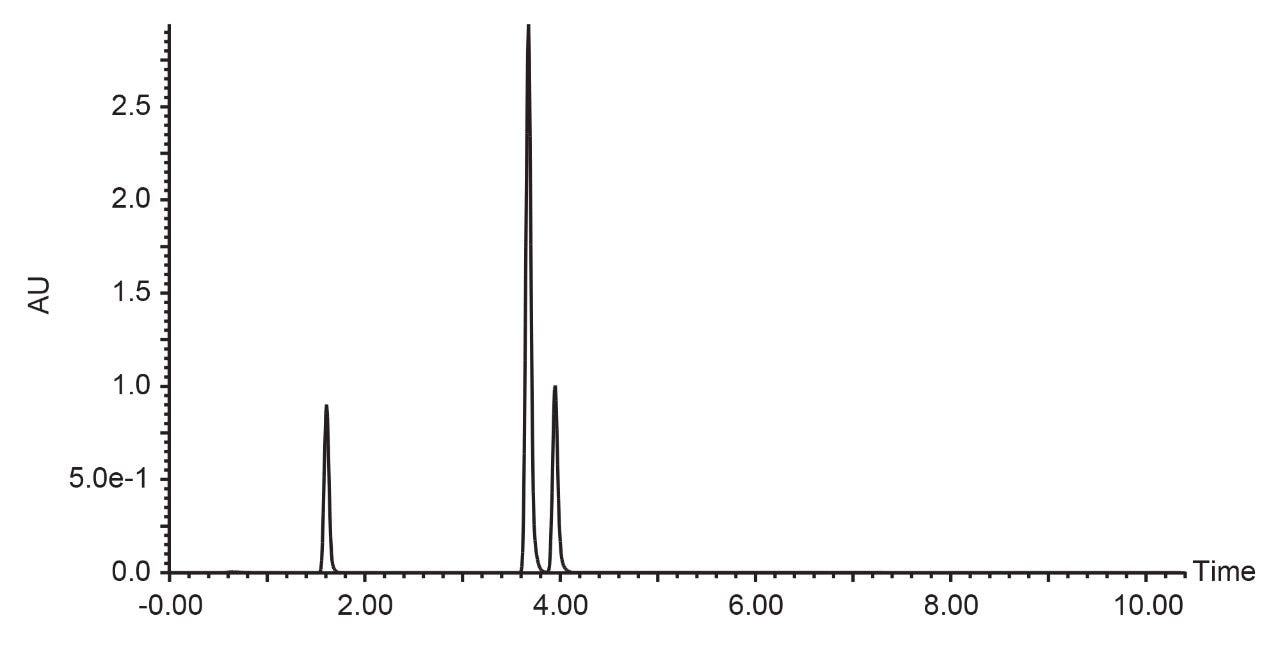
Acetonitrile is often used in purification laboratories as its low viscosity promotes low backpressure even at the high flow rates used in prep chromatography. However, preparative chromatography consumes large amounts of solvents, which has an environmental impact. With the growing trend of green chemistry, more purification labs are evaluating alternative, more environmentally-friendly solvents. Figure 5 illustrates the compound separation using methanol at 24 mL/min.
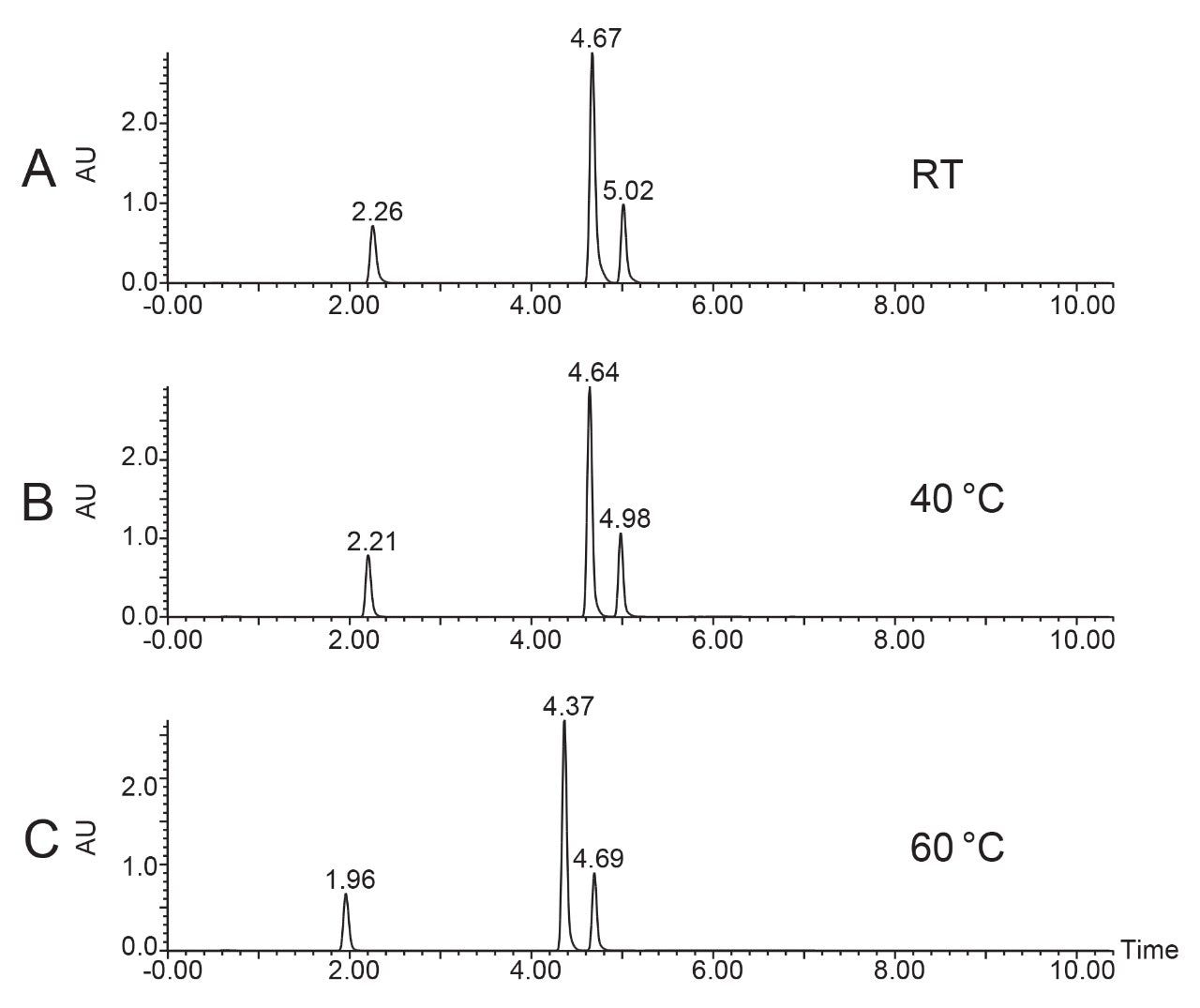
Precision and stability evaluation of the temperature control and system performance at different flow rates and temperatures is shown in Figures 6–9. The results show minimal temperature variation over the course of the chromatographic runs. As expected, increased temperature reduces the system/column back pressure. The temperature and pressure data collected over the course of preparative chromatographic runs also illustrate the reliability of the Timberline oven when configured in the AutoPurification System.
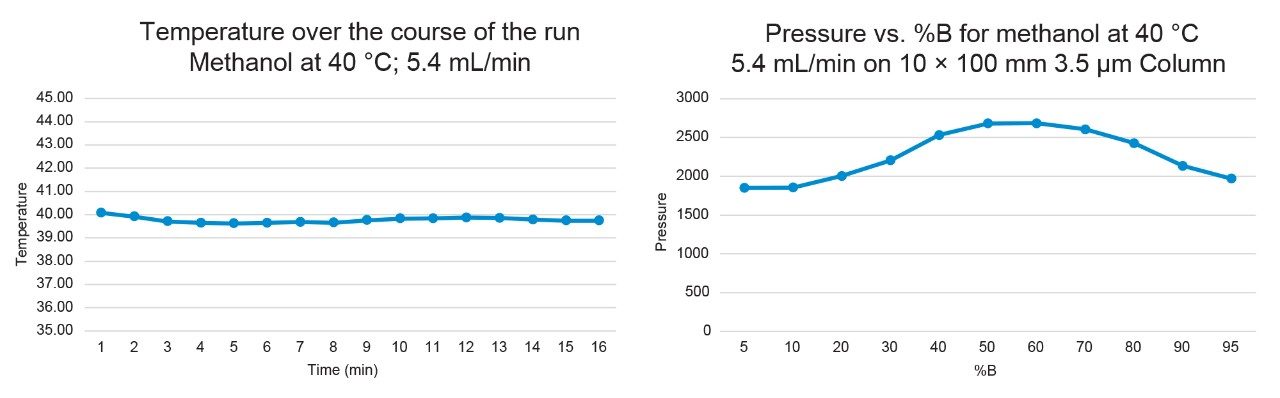
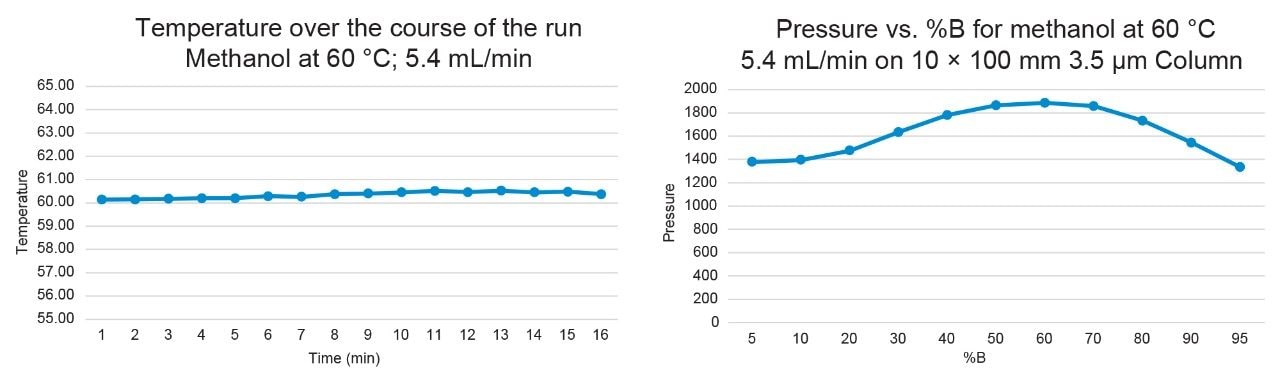
Figures 6–7 demonstrate that the temperature remained highly stable at 5.4 mL/min flow rate, fluctuating by less than 0.5 °C at 40 °C and 60 °C throughout the runs.
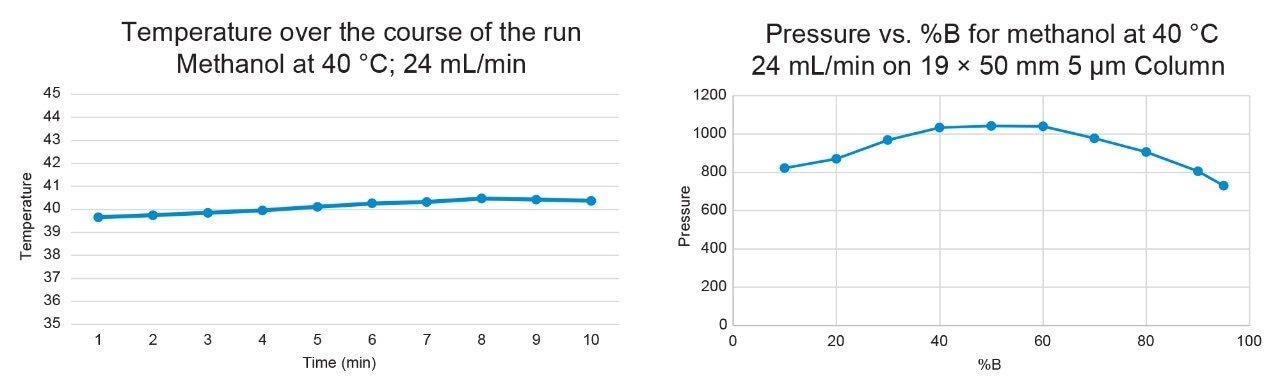
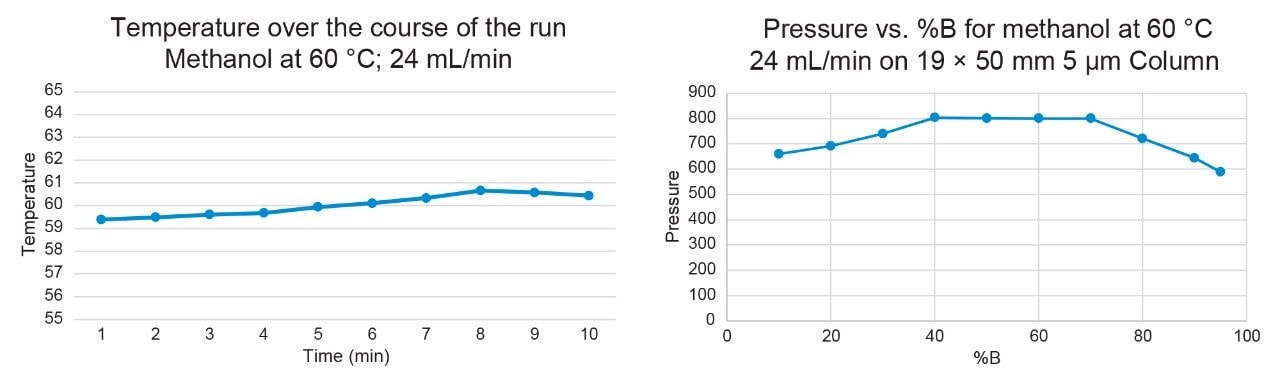
Figures 8–9 demonstrate that the temperature remained highly stable at the 24 mL/min flow rate, fluctuating by less than 1°C at 40 °C and 60 °C throughout the run.
Timberline’s TL-105 D column oven configured with the Waters AutoPurification System provides precise and consistent temperature control when scaling from analytical to preparative methods. This significantly reduces the need for method redevelopment at the prep scale, enhances chromatographic efficiency and reproducibility and delivers predictable and reliable outcomes for various applications including small and large molecules such as oligonucleotides and peptides isolations.
This combined solution supports the growing demand for green chemistry by enabling the substitution of acetonitrile with methanol. Enhanced temperature control also lowers system backpressure, allowing the use of smaller particle size, larger dimension Waters preparative OBD columns. This results in faster separations, increased loading capacity, reduced solvent consumption, shorter fraction dry-down times, and lower waste disposal costs. These benefits contribute to a more environmentally sustainable and economically efficient purification process.
720008874, June 2025