Mitigation of Non-Specific Adsorption During Column Conditioning for an Oligonucleotide HILIC Analysis
Abstract
Oligonucleotides, the short strands of nucleic acids that are pivotal in genetic research and therapeutic applications, require precise analytical techniques for their characterization. Hydrophilic Interaction Liquid Chromatography (HILIC) has emerged as a powerful alternative to traditional ion-pair reversed phase chromatography for oligonucleotides analysis (<35 mer). HILIC offers several advantages, including reduced mobile phase cost, lower toxicity, and improved stability, making it a cost-effective and environmentally friendly option. However, oligonucleotides are known to interact with metal surfaces, which can lead to non-specific adsorption onto both the column and LC hardware and negatively impact recovery and peak shape. Historically, one solution to non-specific adsorption was passivation, which is the process of masking active sites of metallic surfaces, but this is time-consuming and not longstanding. Another solution to address non-specific adsorption is the use of MaxPeak High Performance Surfaces (HPS) Technology, which offers improvements in separation and detection of metal-sensitive analytes. This application note focuses on the analysis of oligonucleotides using HILIC conditions to compare traditional stainless-steel High Performance Liquid Chromatography (HPLC) Systems and columns with systems and columns employing MaxPeak HPS Technology.
Benefits
- Increased recovery of oligonucleotides with “out-of-the-box" performance using MaxPeak HPS Technology on both system and column without the need for passivation or ion-pairing reagents
- HILIC separation of oligonucleotides uses fewer toxic solvents, contributing to a greener laboratory environment while also reducing operational costs
- Improved robustness and stability for HILIC separation of oligonucleotides with the Alliance™ iS Bio HPLC System and the MaxPeak HPS Column
Introduction
HILIC has emerged as a powerful alternative to traditional ion-pair reversed phase chromatography for oligonucleotide analysis. HILIC offers several advantages, including reduced mobile phase cost, lower toxicity, and improved stability, making it a cost-effective and environmentally friendly option. However, oligonucleotides are known to interact with metal surfaces, which can lead to non-specific adsorption onto the column hardware and system tubing, thus affecting recovery and peak shape. Oligonucleotides are a class of compounds that commonly exhibit non-specific adsorption due to the electron-rich phosphodiester backbone. Another solution to this non-specific adsorption is passivation, which is the process of masking active sites of metallic surfaces, but this is time-consuming, and not longstanding. To mitigate this, Waters developed the MaxPeak High Performance Surfaces (HPS) Technology, which reduces the non-specific adsorption for metal-sensitive analytes. This approach not only enhances the accuracy of oligonucleotide detection but also contributes to the advancement of gene therapy and personalized medicine by providing reliable data for the development of targeted treatments. The following comparative study will focus on the analysis of oligonucleotides using HILIC conditions in tandem with a system, the Alliance iS Bio HPLC System, and column that incorporate MaxPeak HPS Technology.
Experimental
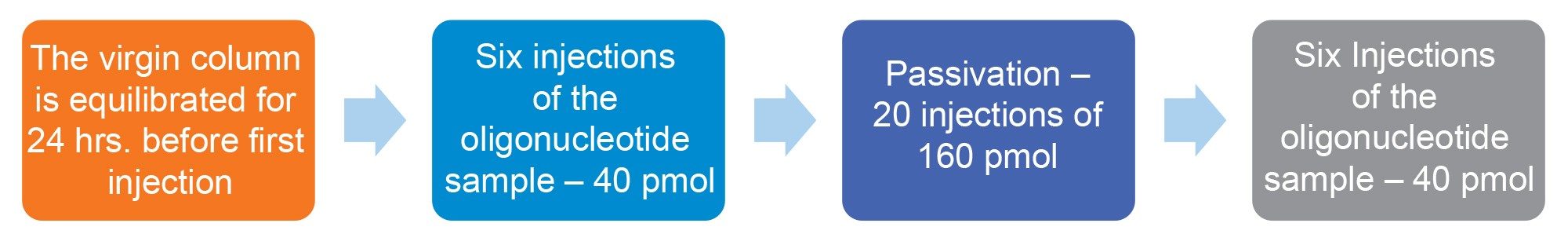
Recovery was calculated by comparing oligonucleotide response (peak area) before and after passivation. The sequence of injections was: six of the oligonucleotide standard (40 pmol of sample on column), followed by 20 injections of the higher concentration passivation solution (160 pmol of the passivation sample on column), and then six of the standard injections (40 pmol of sample on column). The sequence was performed to ensure the column was fully passivated and was verified by calculating the percent relative standard deviation of the area of the last six injections. Low %RSDs (<2%) verified passivation. The first injection after passivation was used to calculate recovery.
This formula was used to calculate the recovery: Recovery (%) = peak area / peak area (Injection 1 after passivation) * 100%
The key difference between the two HPLC systems (the Alliance™ iS HPLC System and the Alliance iS Bio HPLC Systems) lies in the presence of MaxPeak HPS Technology, which is designed to mitigate undesired interactions between analytes and metal surfaces. Another difference between the two HPLC systems is the Alliance iS Bio HPLC System contains MP35N®, which is corrosion resistant. This can significantly improve the recovery, peak shape, and reproducibility of metal-sensitive analytes, such as oligonucleotides. Using the same packing material in both columns ensures that any observed differences in performance could be attributed to the MaxPeak HPS Technology itself. This setup allows for a clear comparison of how MaxPeak HPS Technology enhances the analysis of oligonucleotides.
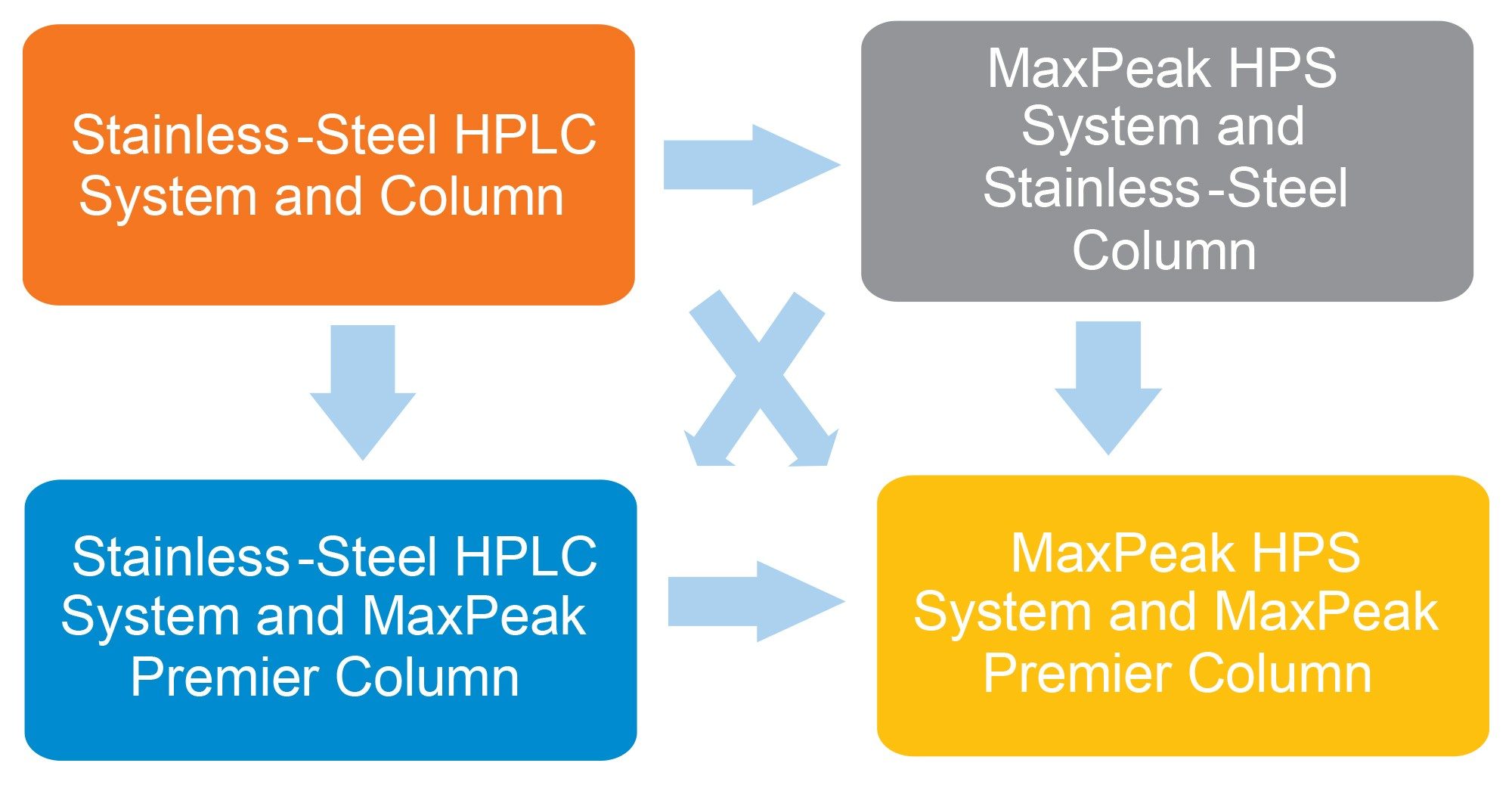
By comparing the responses of the oligonucleotide peaks before and after passivation, you can effectively measure the technology’s efficiency in improving analyte recovery. The sequence of injections - six of the oligonucleotide standard, followed by 20 of the passivation solution, and then another six of the standard - provides a robust dataset for assessing the maximum response. The testing matrix shown in Figure 1 illustrates the different combinations of LC systems and columns that will be used to evaluate the impact of MaxPeak HPS Technology on the analysis of oligonucleotides using HILIC conditions.
Standard
One vial of Waters MassPREP™ OST Standard (p/n: 186004135) was reconstituted in 500 µL of 20:80 (v/v) water:acetonitrile to provide 40 pmol of sample on column.
Passivation Sample
Two vials of Waters MassPREP OST Standard (p/n: 186004135) were reconstituted in 500 µL of 20:80 (v/v) water:acetonitrile to provide 160 pmol of the passivation sample on column.
The sequence, Figure 2, was followed for each oligonucleotide analysis performed to provide consistent data across each of the different combinations of HPLC systems and columns.
LC Conditions
|
LC systems: |
1. Alliance iS HPLC System 2. Alliance iS Bio HPLC System |
|
Detection: |
TUV Detector with 10 mm analytical flow cell |
|
Wavelength: |
260 nm |
|
Sampling rate: |
5 Hz |
|
Vials: |
LCGC certified clear glass 12 x 32 mm screw neck vial, total recovery with cap and Preslit PTFE/Silicone septum 1 mL Volume (p/n: 186000384C) |
|
Column(s): |
1. XBridge™ Premier BEH™ Amide Column, 2.5 µm, 4.6 x 100 mm (p/n: 186009936) 2. XBridge BEH Amide Column, 2.5 µm, 4.6 x 100 mm (p/n: 186006099) |
|
Column temperature: |
60 °C |
|
Sample temperature: |
10 °C |
|
Standard injection volume: |
10 µL (4 pmol/µL) |
|
Passivation injection volume: |
20 µL (8 pmol/µL) |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
50 mM ammonium acetate, pH=6.7 |
|
Sample manager wash: |
20:80 (v/v) Water:Acetonitrile |
|
Sample manager purge: |
20:80 (v/v) Water:Acetonitrile |
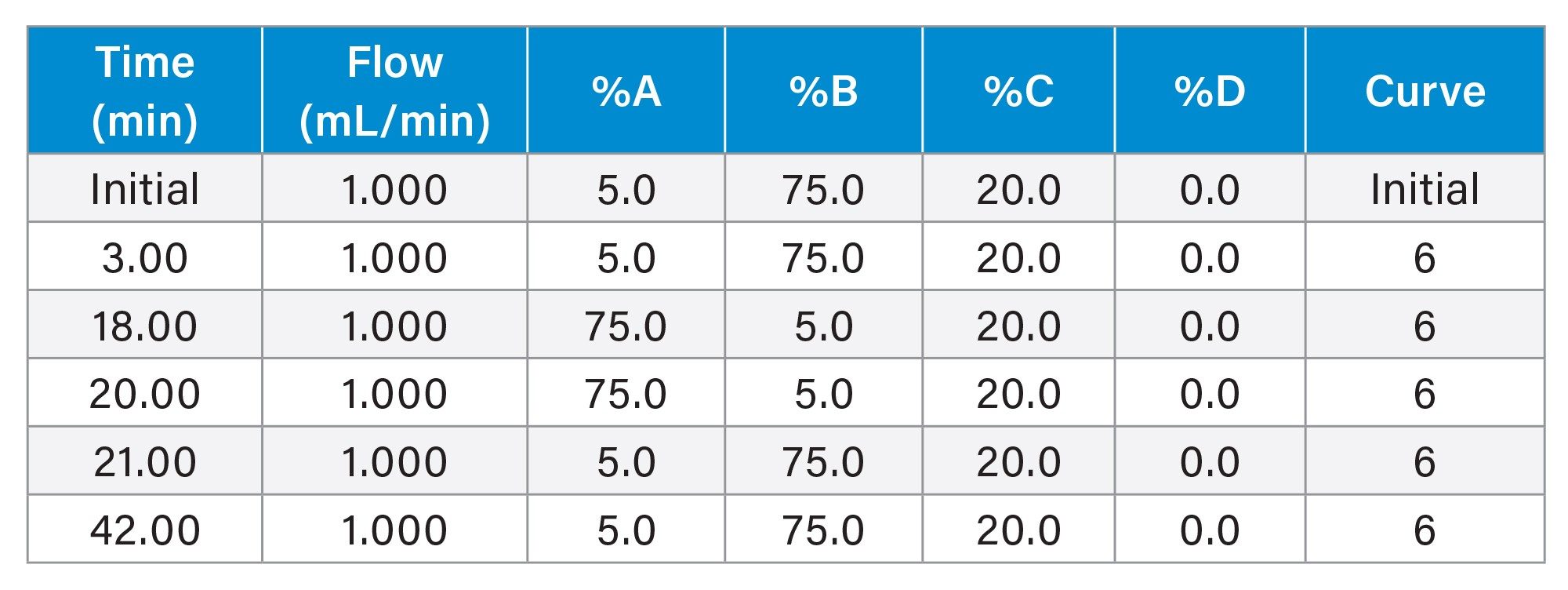
Gradient Table
Data Management
|
Chromatography data system: |
Empower™ 3.7.0 |
Results and Discussion
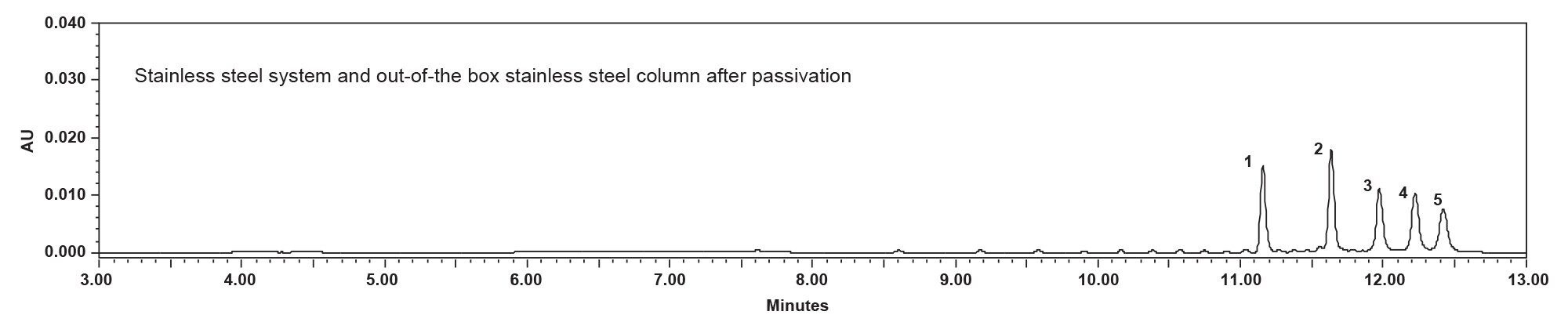
The experiment utilized a stainless-steel HPLC System, specifically the Alliance iS HPLC System, in conjunction with a stainless steel XBridge BEH Amide Column. Under these conditions, observations align with common chromatographic behavior where new, unused columns often show lower peak areas for oligonucleotides due to non-specific adsorption on unpassivated metal surfaces. This phenomenon was consistently observed across the first six injections (Figure 3) before passivation. It has been reported in the literature that phosphorylated compounds are retained on the stainless steel of the HPLC System due to their phosphodiester backbone as in the case of oligonucleotides.4 Non-specific adsorption is the result of interactions between negatively charged analytes or highly acidic analytes and the relatively electron depleted metal surfaces of the LC and column.
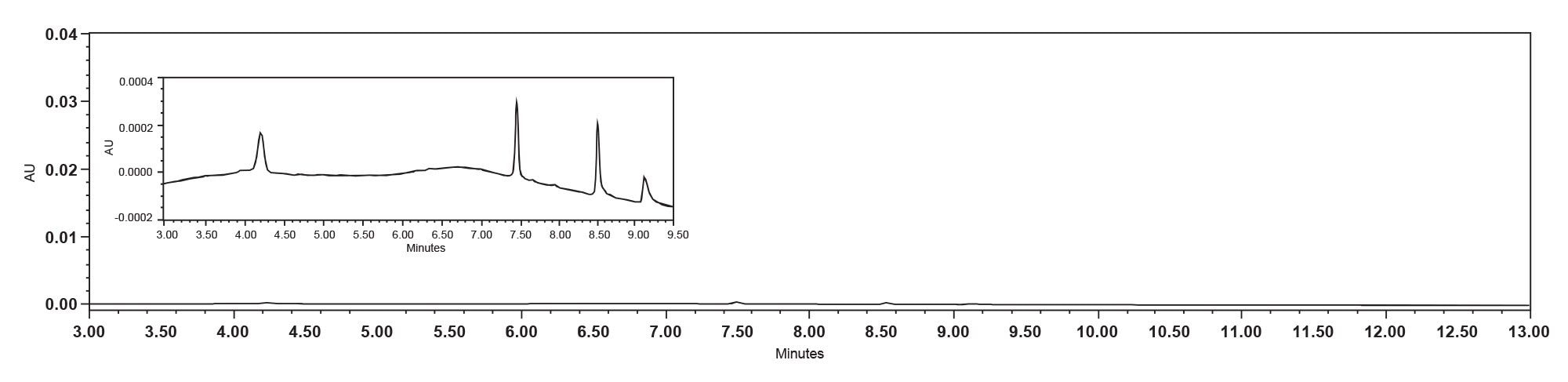
However, passivation, which involves treating the metallic surfaces to mask active sites, appears to have had a positive effect on the analysis of oligonucleotides in the stainless-steel HPLC System. The increase in peak areas and retention times post-passivation indicates a reduction in non-specific adsorption, which is crucial for accurate recovery and chromatographic analysis (Figure 4).
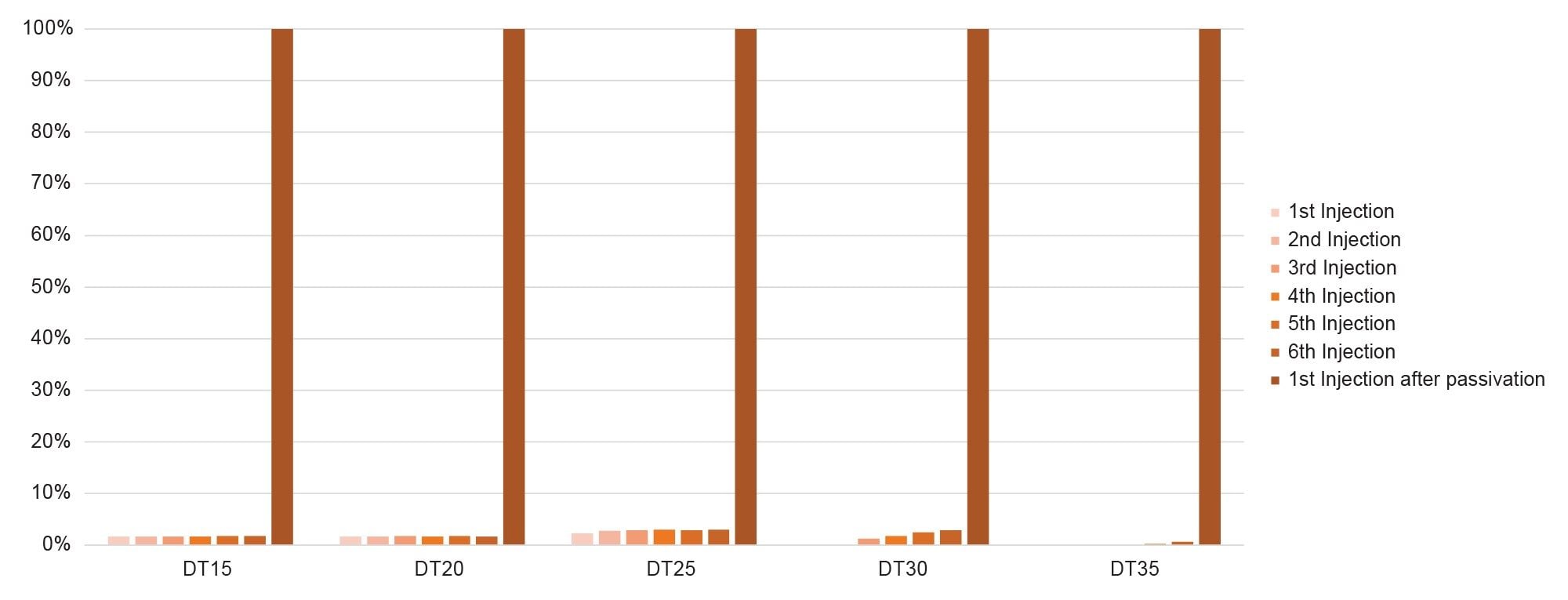
If the previous results were analyzed by percent recovery, the reduction in nonspecific adsorption can be quantified and graphed.
The application of the passivation technique can mitigate this issue, as evidenced by the complete recovery observed in Figure 5. This demonstrates the effectiveness of surface passivation in preventing non-specific adsorption, thereby ensuring the integrity, and accuracy of the assay result, but passivation is time-consuming and not longstanding.
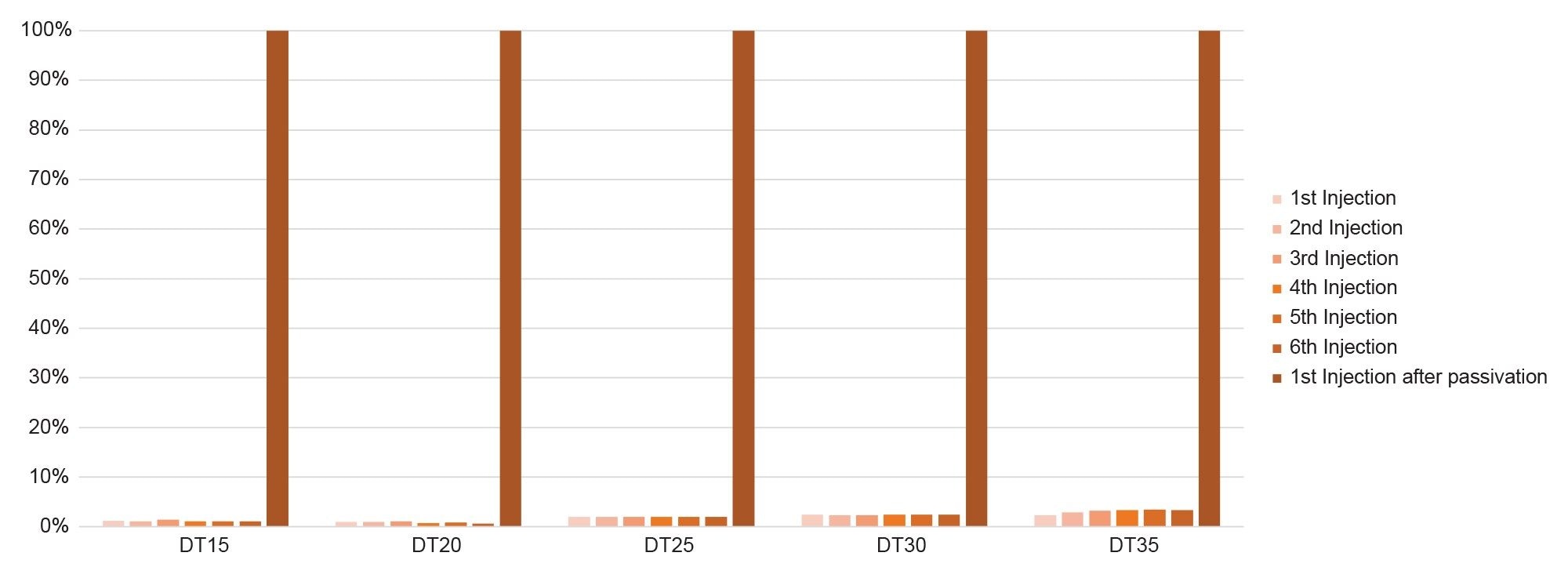
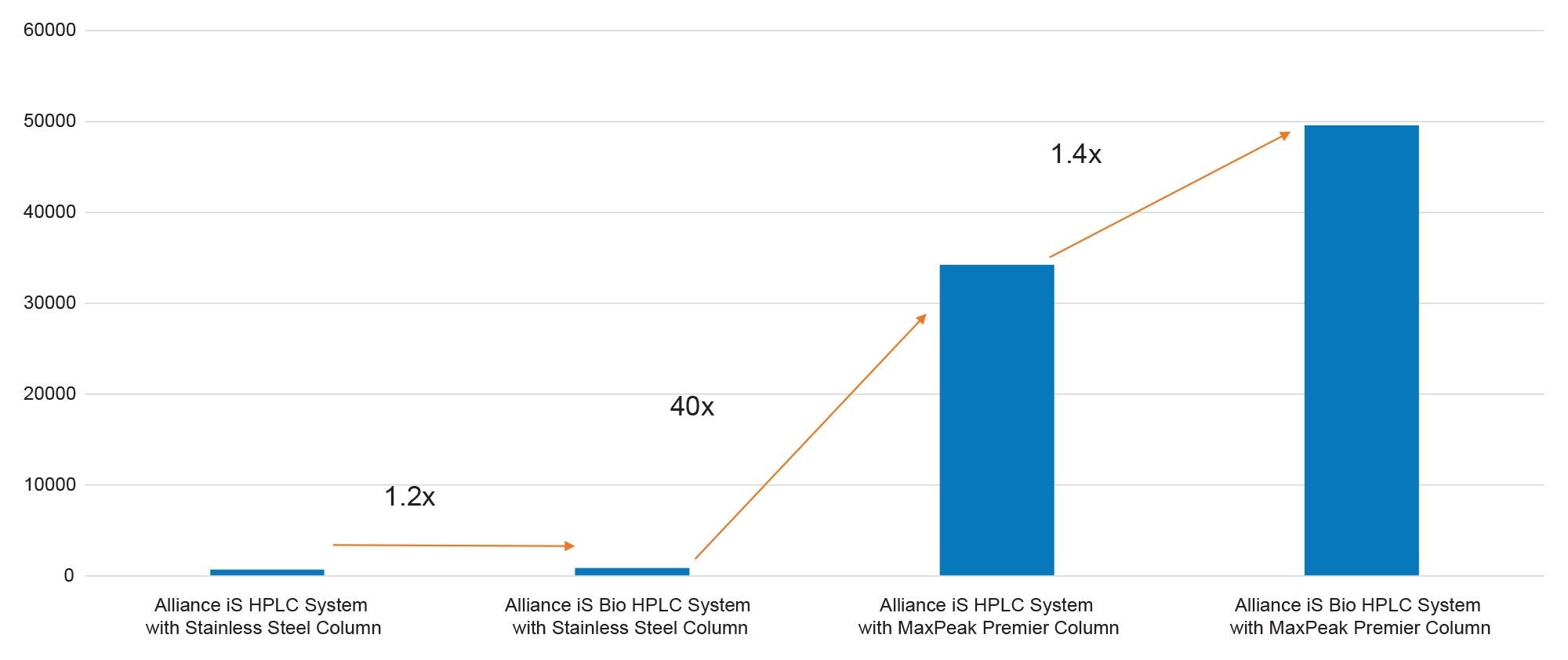
When a MaxPeak HPS Premier Column was paired with the Alliance iS HPLC System (stainless-steel HPLC system), the result was significantly improved in that the percent recovery increased, but peak area reproducibility was still problematic. Initial injections on the new column yielded a higher recovery when compared to the stainless-steel column (Figure 6) but an increasing percent recovery was observed and only achieved an average of ~57% recovery for the non-passivated column. This result indicates that the MaxPeak Premier Column effectively eliminates the non-specific adsorption on the column but that the non-specific adsorption was still occurring on the stainless-steel HPLC system, which affected the reproducibility and recovery of the oligonucleotides.

To evaluate the reduction in non-specific binding using an inert LC, the Alliance iS Bio HPLC System was paired with a stainless-steel XBridge BEH Amide Column. As can be seen in Figure 7, the result was similar to the result that was obtained with an Alliance iS HPLC System and a stainless-steel column. This result demonstrates the importance of having MaxPeak HPS Technology on the column, and that most of the nonspecific adsorption that is affecting the chromatography is still occurring on the column.
The experiment with the Alliance iS Bio HPLC System paired with a MaxPeak Premier Column shows highly improved recovery for the initial injections that have no passivation. The initial recovery on average was 98% (Figure 8), which is a substantial improvement, and highlights the potential of MaxPeak HPS Technology in reducing non-specific adsorption. Consistency in response over multiple injections is a critical factor in analytical methods, especially in fields like pharmaceuticals where precision is paramount. As was seen in the area percent standard deviation (RSD) for first six injections before passivation on a new column of MassPREP OST Standard, the reproducibility improves significantly with the incorporation of MaxPeak HPS Technology. The improved retention, response, and peak shape observed in the chromatogram (Figure 9) further supports the combination's effectiveness. This data not only underscores the reliability of the system and column pairing but also shows that incorporating MaxPeak HPS Technology is valuable for analyses of compounds that are prone to or have the potential for non-specific adsorption to the LC flow path and column.
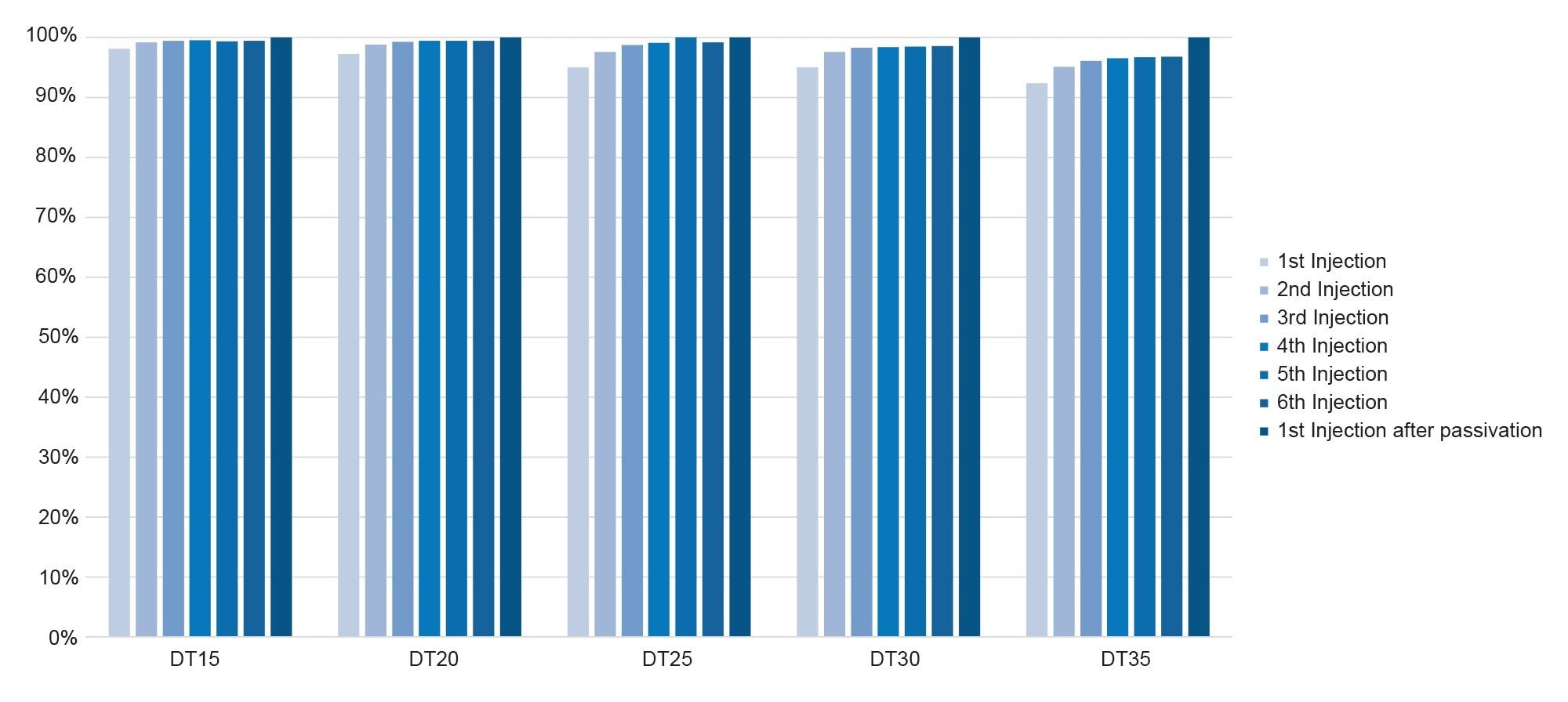
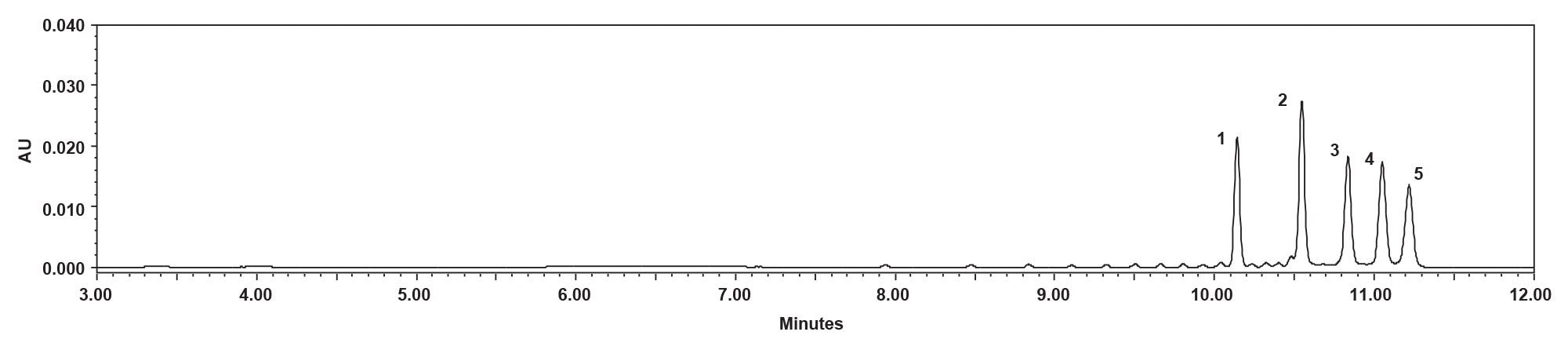
The differences in response observed between a stainless-steel system and a system with MaxPeak HPS Technology (Figure 10) was significant; the magnitude of the difference in response may be greater than previously observed because of the passive preheater. The passive preheater with MaxPeak HPS Technology may have more impact on the chromatography than the active preheater with MaxPeak HPS Technology because of the differences in tubing dimensions, the passive preheater is significantly longer.5
Conclusion
Oligonucleotides are susceptible to non-specific adsorption in LC-based analyses and often require column conditioning to ensure repeatable performance, especially without ion-pairing reagents. In this study, it was demonstrated that columns engineered with MaxPeak HPS Technology performed better in terms of “out-of-box" performance with respect to analyte recovery and passivation when compared to their stainless-steel counterparts. In summary, the combination of MaxPeak HPS Technology in both the column and system enabled higher analyte recoveries and reduced the need for excessive passivation and offered a significant advancement in chromatographic performance, which is crucial for analytical precision and reproducibility.
References
- Eric S. Grumbach and Kenneth J. Fountain. Comprehensive Guide to HILIC Hydrophilic Interaction Chromatography. Waters Corporation, 2010.
- Martin Gilar, Mathew DeLano, Fabrice Gritti. Mitigation of Analyte Loss on Metal Surfaces in Liquid Chromatography. J Chrom A. 2021, 1650, 462247.
- Honorine Lardeux, Alexander Goyon, Kelly Zhang, Jennifer M. Nguyen, Matthew A. Lauber, Davy Guillarme, Valentina D’Atri. The Impact of Low Adsorption Surfaces for the Analysis of DNA and RNA Oligonucleotides, J Chrom A. 2022, 1677, 463324.
- Tuytten, R., Lemière, F., Witters, E., Van Dongen, W., Slegers, H., Newton, R. P., Van Onckelen, H., & Esmans, E. L. Stainless steel electrospray probe: a dead end for phosphorylated organic compounds? J Chrom A. 1104(1–2), 209–21. 2006.
- Kathryn Brennan, Mary Trudeau, and Paul D. Rainville. Utilization of the ACQUITY Premier System and Column for Improved Oligonucleotide Bioanalytical Chromatographic Performance. Waters Application Note. 720007119. January, 2021.
720008694, February 2025