High-Resolution Separations of Single and Double-Stranded Nucleic Acids Using Strong Anion-Exchange Chromatography
Abraham S. Finny, Balasubrahmanyam Addepalli, Matthew A. Lauber
Waters Corporation, United States
Published on November 18, 2025
Abstract
Reliable separation of nucleic acid molecules is essential for advancing gene therapeutics and ensuring both product quality and regulatory compliance. Depending on the therapeutic modality and mechanism of action, nucleic acids must be accurately characterized across a broad size range. This application note presents an optimized anion-exchange liquid chromatography (AEX) method utilizing the Waters Protein-Pak™ Hi-Res Q Column, capable of achieving high-resolution separation from 10-nucleotide single-stranded DNA (ssDNA) to 15,000 base pair double-stranded DNA (dsDNA). The use of well-defined nucleic acid size standards helps assess system suitability prior to drug substance analysis. The method uses a Tris-HCI buffer (pH 9.0) with 5% (w/v) urea and a sodium chloride gradient to resolve multiple components of nucleic acid standards. These optimized conditions effectively minimize secondary structure interference and fully exploit charge-based interactions, enhancing selectivity and resolution. This robust, scalable, and automatable approach offers a compelling alternative to traditional electrophoresis, delivering reproducible chromatograms with baseline resolution. It enables precise, size-based analysis critical to biopharmaceutical development and regulatory submissions.
Benefits
- Enhanced resolution on the Waters Protein-Pak Hi Res Q Column for separating single and double-stranded DNA fragments across a broad size range from 10-mer to 15 Kbp.
- Utilization of a urea-supplemented NaCl gradient to reduce complementary sequence-dependent effects and improve charge-based selectivity in anion-exchange separations.
- Streamlined, single-flow-rate method offering rapid deployability and ease of implementation for nucleic acid analysis.
Introduction
Gene therapy and vaccine technologies rely on manipulating genetic material to achieve therapeutic outcomes, such as correcting defective genes or eliciting immune responses. Plasmids serve as essential vectors in these applications, either directly as therapeutics or for producing viral genomes, such as adeno-associated viruses (AAVs).1 Regulatory frameworks, including those from the U.S. Food and Drug Administration (FDA), mandate a comprehensive characterization of these materials].2 Nucleic acid standards, such as DNA ladders, including single (ss) and double-stranded (ds) DNA segments, serve as reference materials to assess system suitability before analyzing real samples by chromatographic techniques. Conventional agarose or polyacrylamide gel electrophoresis, while effective, suffers from limitations in throughput, quantitation, and fraction collection for downstream and automated studies.
AEX addresses analytical challenges by exploiting differences in negative charge density arising from phosphate groups in the DNA backbone. For linear dsDNA fragments, charge correlates strongly with length, enabling size-based elution under increasing ionic strength. However, sequence composition, particularly adenine-thymine (A-T) content, can influence retention due to secondary interactions or structural variations.3 To mitigate this, the inclusion of urea as a mild denaturant disrupts hydrogen bonding, promoting more uniform unfolding and exposure of charged residues. This application note describes an optimized AEX method using the Waters Protein-Pak Hi Res Q Strong Anion-Exchange Column with an ACQUITY™ Premier LC System. A sodium chloride (NaCl) gradient in urea-containing Tris buffer at pH 9.0 facilitates the separation of both ss and dsDNA segment ladders, offering superior resolution and scalability for biopharmaceutical workflows. Urea further minimizes non-specific binding, ensuring that electrostatic forces primarily govern analyte retention and elution. Symmetrical peaks and excellent separations can thereby be achieved even when an analyte contains secondary structures, such as hairpins or A-T-rich regions, which could otherwise introduce sequence-dependent retention biases and compromise size-based resolution. It is demonstrated that Waters Protein-Pak Hi Res Q Anion-Exchange Columns provide strong separation capabilities independent of strand type (ss vs ds) or size (10-mer to 15,000 bp), making them highly suitable for size-variant analysis of nucleic acid therapeutics.
Experimental
Multiple nucleic acid standards were selected to assess the AEX method's capability in resolving ss and dsDNA by size:
LC Conditions
Note on Flow Cell Selection: A 5 mm titanium flow cell (ACQUITY PDA Detector, Waters p/n: 205000613) was used to minimize analyte adsorptive interactions during analysis. Prior experiences with SEC-UV have demonstrated that the titanium flow cell’s chemical inertness reduces the adsorption of nucleic acids and phosphate species.4
|
LC system: |
ACQUITY Premier LC System with Binary Solvent Manager (BSM, p/n: 186018000) Flow-Through Needle Sample Manager (SM-FTN, p/n: 186018002) Column Manager-A (CM-A, p/n: 186018003) |
|
Detector: |
ACQUITY Premier eLambda PDA Detector (p/n:186018007) with 5 mm Titanium Flow Cell (p/n: 205000613) |
|
Column(s): |
Protein-Pak Hi Res Q Column, 5 µm, 4.6 mm x 100 mm (p/n: 186004931) |
|
Mobile phase A: |
20 mM Tris, 5% (w/v) urea, pH 9.0, 0.1 µm filtered |
|
Mobile phase B: |
20 mM Tris, 5% (w/v) urea, 1.0 M NaCl, pH 9.0, 0.1 µm filtered |
|
Vials: |
TruView™ pH Control LCMS Certified Clear Glass Vials, 12 × 32 mm, Screw Neck (Waters, p/n: 186005663CV) |
|
Column temperature: |
35 °C |
|
Sample temperature: |
5 ± 3 °C |
|
Flow rates: |
0.5 mL/min |
|
Seal wash: |
10% HPLC-grade Methanol / 90% 18.2 MΩ*cm resistivity (Milli-Q®) water (v/v) |
|
Injection volume: |
1 µL (1 Kb Plus DNA Ladder), 2 µL (ssDNA 10 to 60 Ladder and ssDNA 20 to 100 Ladder) |
|
Gradient: |
Refer to the table below |
|
System control & data acquisition: |
Empower™ 3.9.x (tested with 3.9.0) |
|
Detection wavelength: |
260 nm |
|
Sampling rate: |
20 points/sec |
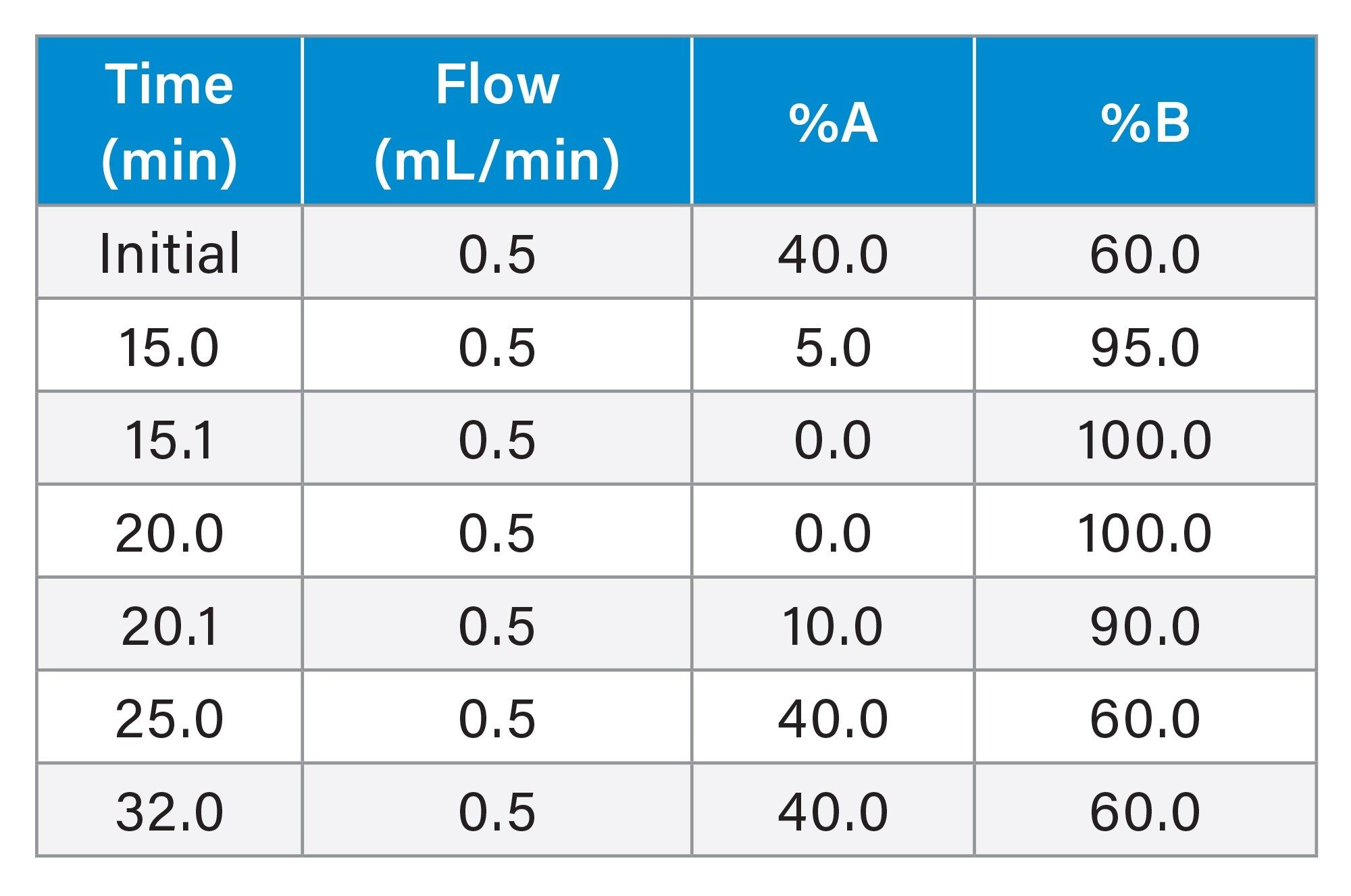
Gradient Table - 0.5 mL/min
Results and Discussion
The performance of the AEX method was first evaluated using two ssDNA ladders. The first ladder contained ssDNA fragments ranging from 10 to 60-mer, and the second ladder contained ssDNA fragments ranging from 20-mer to 100-mer. Separations were conducted on the Protein-Pak Hi Res Q Column using a 20 mM Tris buffer (pH 9.0) containing 5% (w/v) urea with the above-described NaCl gradient (see experimental) at a 0.5 mL/min flow rate (Figure 2).
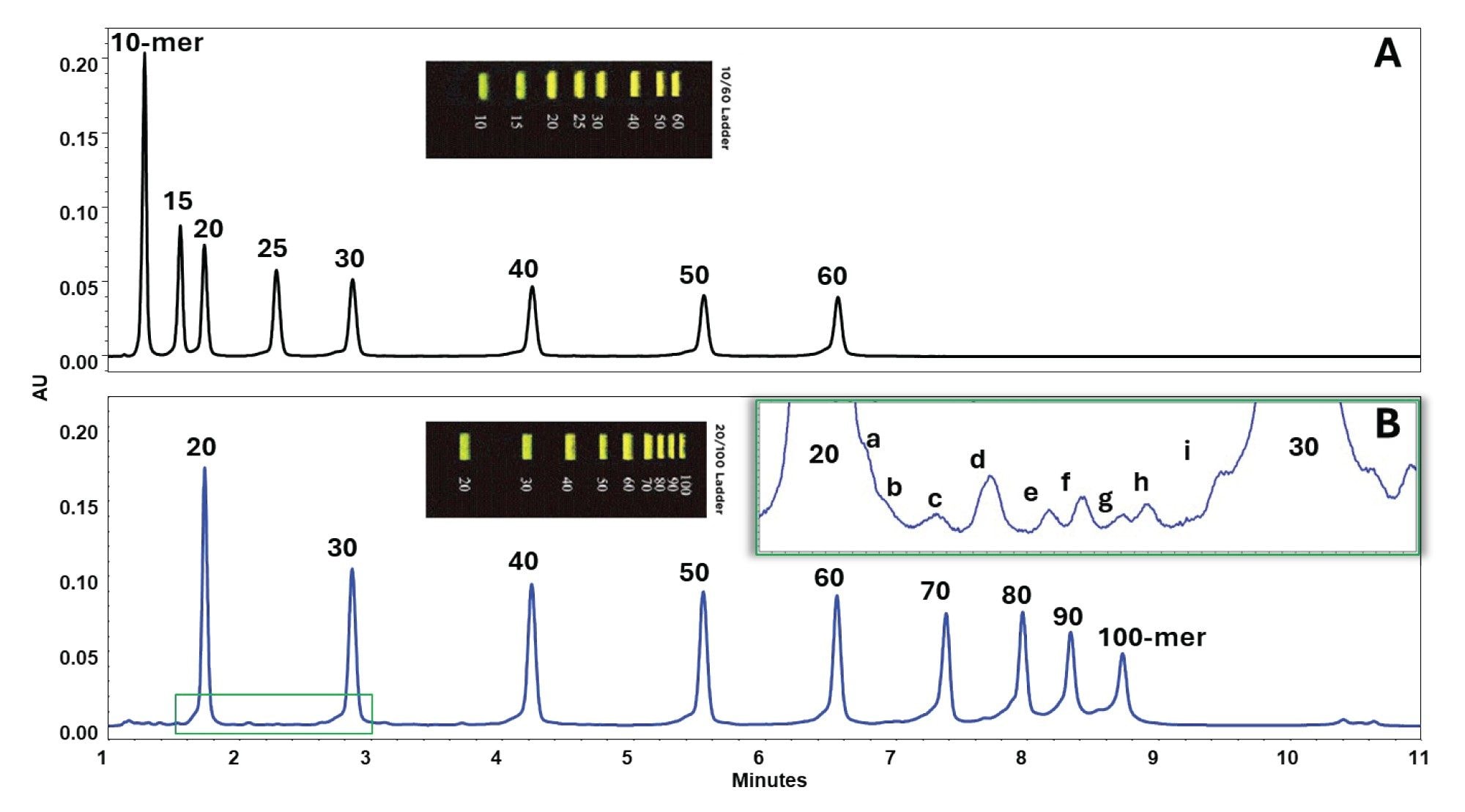
The method achieved clear resolution of discrete peaks, with smaller fragments eluting first due to lower charge density and weaker interactions with the quaternary ammonium stationary phase. Larger fragments, possessing more phosphate groups, required higher NaCl concentrations to desorb from the column.
The performance of the AEX method was further evaluated using a 1 Kbp Plus DNA Ladder, which contains dsDNA fragments from 100 bp to 15,000 bp. Separations were conducted on the Protein-Pak Hi Res Q Column using a 20 mM Tris buffer (pH 9.0) containing 5% (w/v) urea and a NaCl gradient at 0.5 mL/min flow rate (Figure 3).
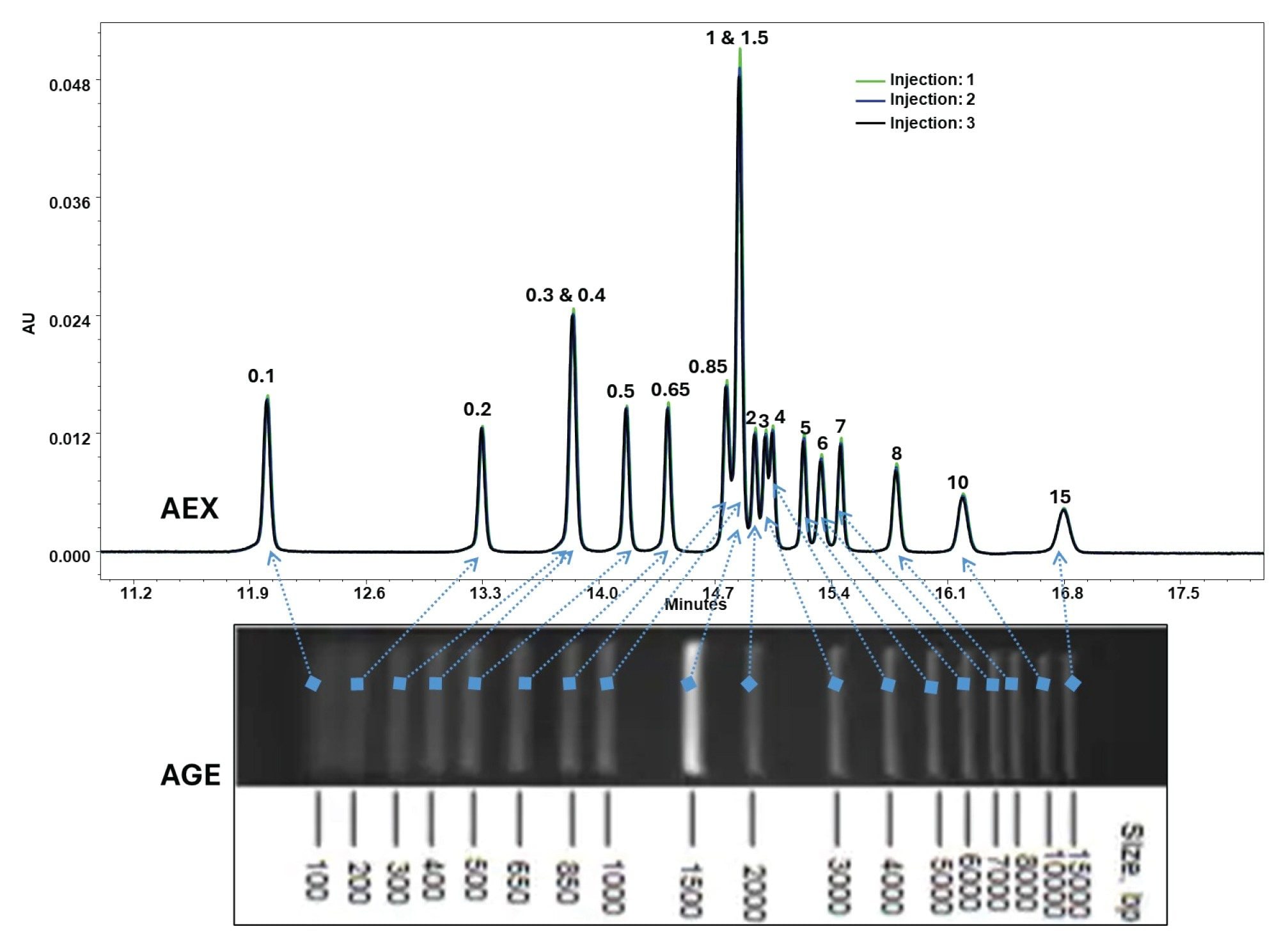
The method demonstrated excellent selectivity, as observed previously; smaller fragments eluted first, and larger fragments, which possess more phosphate groups, required higher NaCl concentrations. Barring the co-elution of the 300 bp/400 bp and the 1 kbp/1.5 kbp species, the rest were well resolved, making each peak prominent and clearly distinguishable.
Recovery and Carryover Studies
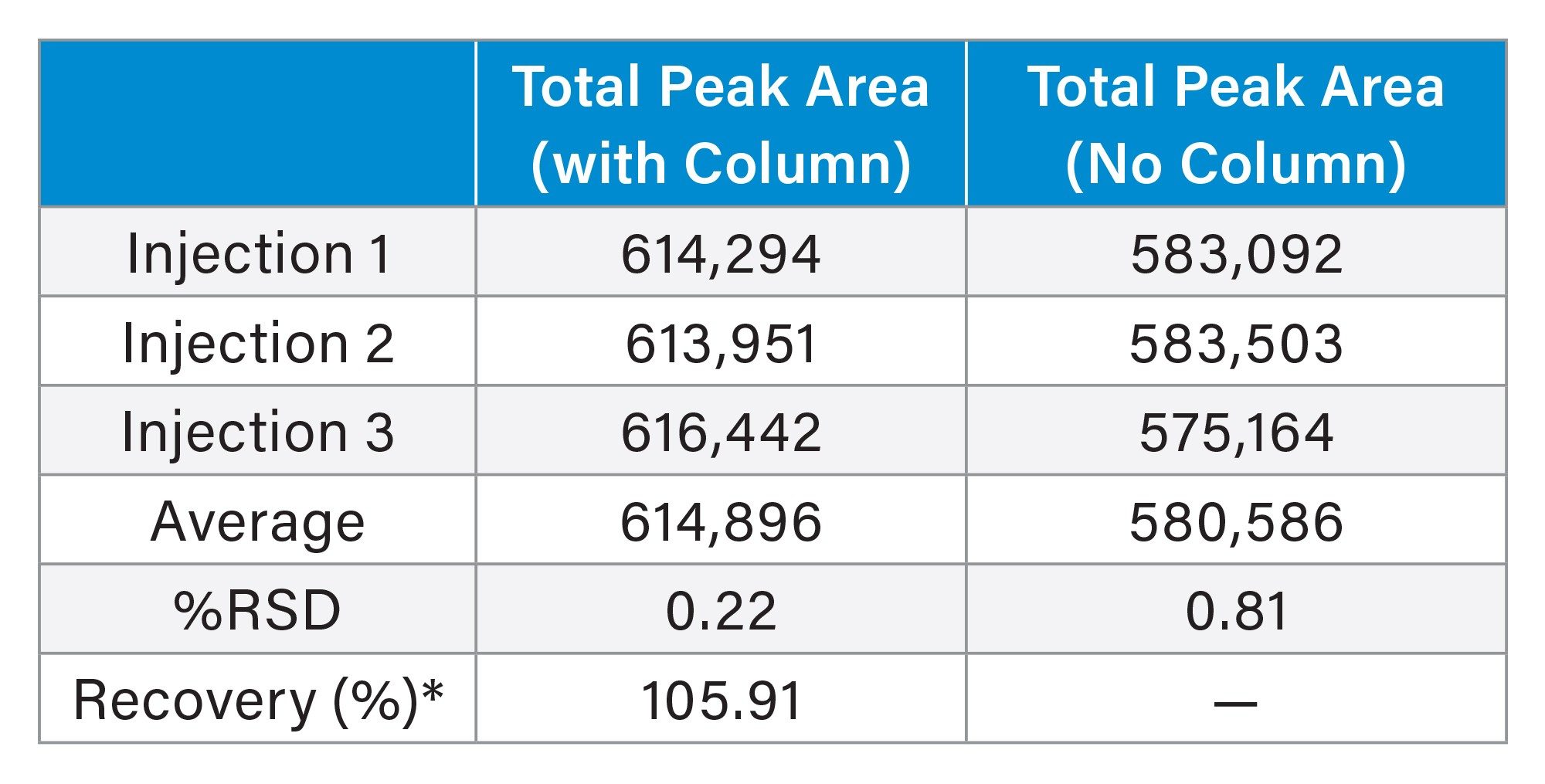
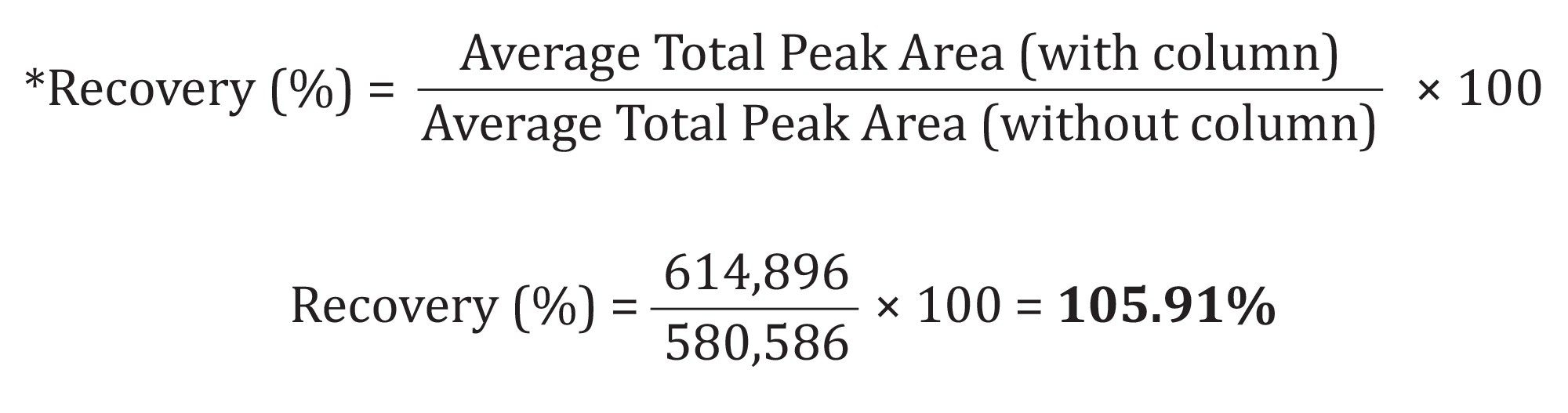
To evaluate whether the injected analytes were fully recovered after chromatographic separation, recovery and carryover tests were performed using the 1 Kb Plus DNA Ladder. Three replicate injections were performed with the column installed, and three were performed without it. The total peak areas were averaged for each condition, as summarized in the table below. The average total peak area obtained with the column (614,896) was comparable to that without the column (580,586), corresponding to an overall recovery of 105.91%. It is assumed that a slightly higher response is observed with a column in place due to a hyperchromic shift. Upon adsorption and desorption from the stationary phase, the nucleic acid analytes might absorb more UV light as a result of increased solvent accessibility. As is, these results indicate this to be a high recovery method. The %RSD values for both conditions were low (0.22% with column and 0.81% without), indicating good injection precision. These results confirm that analyte recovery was complete and that the column did not contribute to sample loss or carryover.
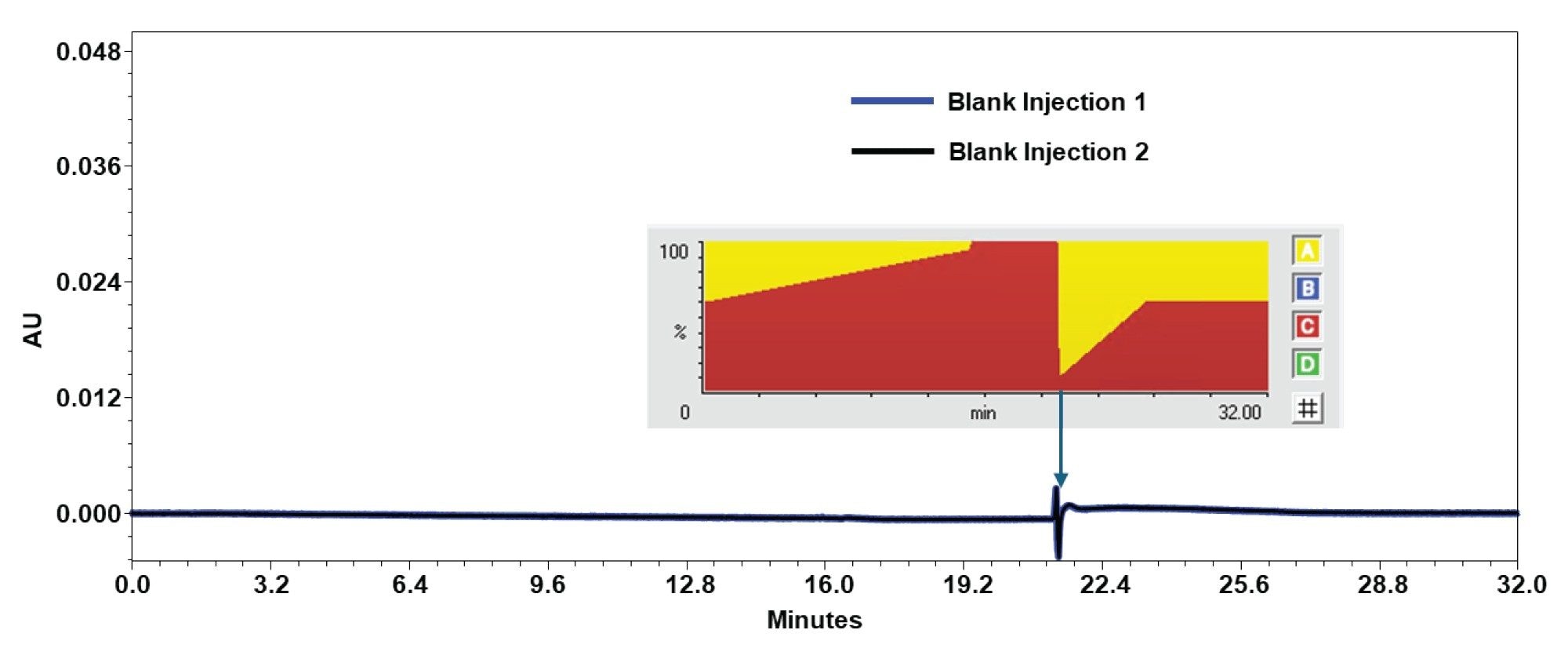
Following the 3 injections of the 1 Kb Plus DNA Ladder, 2 blank injections were performed to test for any adverse carryover effects. As shown in Figure 4, the baselines showed no evidence of potential carryover.
Impact of Urea
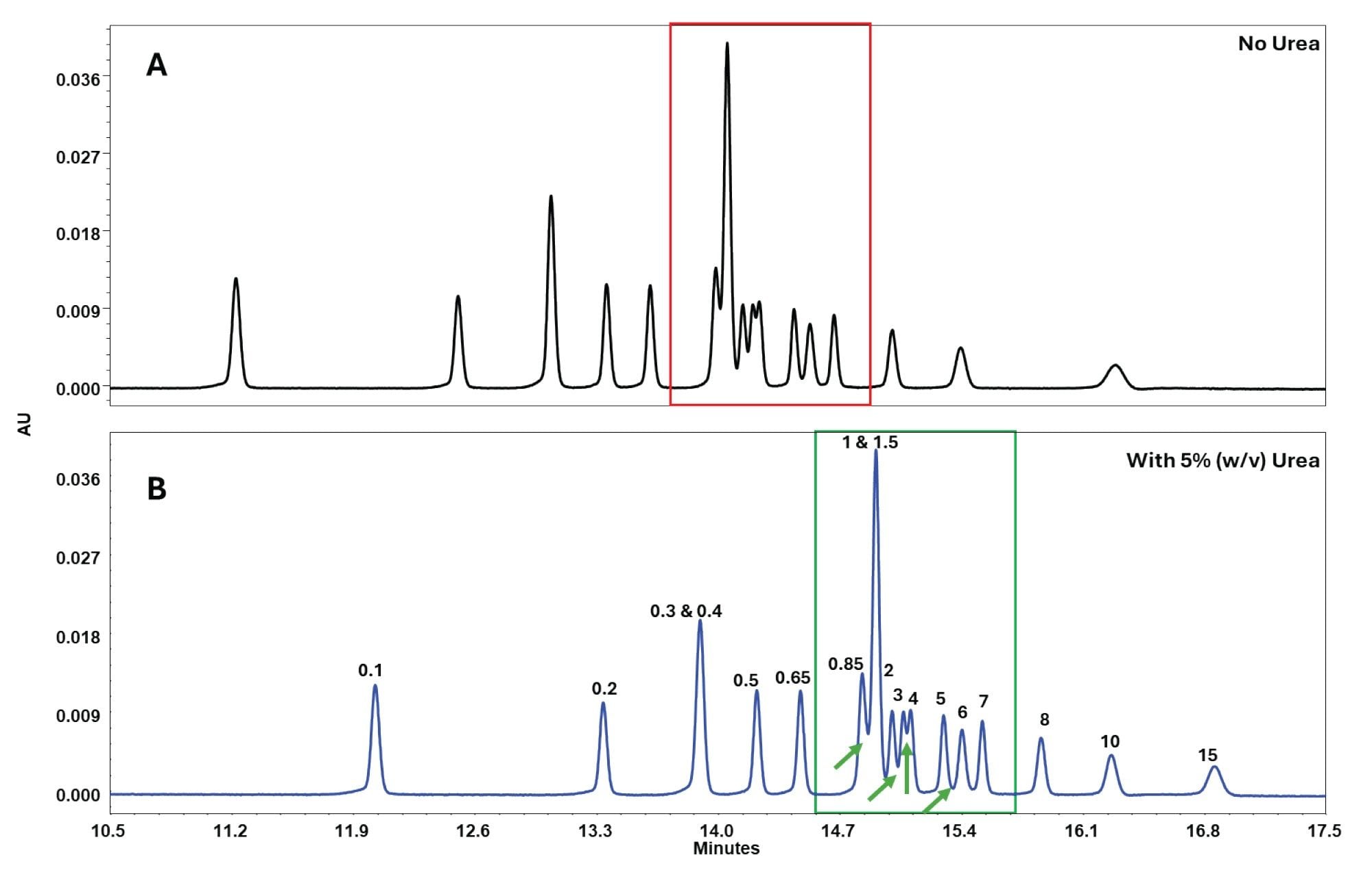
The addition of 5% (w/v) urea to the mobile phases resulted in sharper and more well-resolved peaks with deeper valleys, as shown in Figure 5, leading to improved resolution. In this application note, urea is shown to be effective while working with nucleic acids. However, when working with proteins, it is important to ensure that urea does not denature or cause post-translational modifications under specific conditions. Considerations specific to the analyte are essential.
Conclusion
This application note establishes the efficacy of the Protein-Pak Hi Res Q Column in strong AEX methods for separating a wide range of DNA fragments, ranging from ssDNA of 10-100mer range to dsDNA of 100 bp–15,000 bp. The urea-enhanced NaCl gradient at 0.5 mL/min optimized charge-based selectivity while suppressing secondary interactions, thereby delivering high-resolution chromatograms with exceptional sample recovery with no carryover. Besides enhancing precision in nucleic acid characterization for gene therapy and vaccine manufacturing, this efficient, scalable alternative to gel-based techniques can also facilitate the collection of peaks of interest for downstream studies. Furthermore, by surpassing the limitations of traditional gel electrophoresis techniques, such as low throughput and challenges in quantitation and automation, this AEX approach offers a scalable, easily transferable, and efficient alternative that integrates seamlessly into biopharmaceutical workflows.
References
- X. Sun, S. Setrerrahmane, C. Li, J. Hu, and H. Xu. Nucleic acid drugs: recent progress and future perspectives. Signal Transduction and Targeted Therapy, vol. 9, no. 1. Springer Nature, pp. 1–31, Dec. 01, 2024. doi: 10.1038/s41392-024-02035-4.
- FDA. Cellular & Gene Therapy Guidances | FDA. Accessed: Oct. 27, 2025. [Online]. Available: https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances.
- H. Yamakawa, K. ich Higashino, and O. Ohara. Sequence-dependent DNA separation by anion-exchange high-performance liquid chromatography. Anal. Biochem., vol. 240, no. 2, pp. 242–250, Sep. 1996, doi: 10.1006/abio.1996.0354.
- A. S. Finny, C. Reidy, B. Addepalli, and M. Lauber. High-Resolution Size Exclusion Chromatography of Megadalton-Sized DNA Vectors and Plasmids Using Waters GTxResolve 2000 Å SEC Columns. Waters Application Note. 720008847. 2025
720009136, November 2025