Application Brief
This is an Application Brief and does not contain a detailed Experimental section.
Philip Wuthrich, Stephan M. Koza, Stephen J. Shiner
Waters Corporation, United States
Published on September 24 2025
This is an Application Brief and does not contain a detailed Experimental section.
In this Application Brief, consistent size-based separations of peptides and small proteins when transferring methods from UHPLC to HPLC have been demonstrated with SEC 125 Å, MaxPeak™ Premier Columns.
Waters ACQUITY™ Premier Systems in combination with ACQUITY 125 Å SEC, MaxPeak Premier 1.7 µm Columns, enable highly efficient size-based separations, reducing run times as well as sample and eluent consumption.1 The speed and efficiency achieved with UHPLC provide clear advantages in R&D and analytical development labs where sample volumes may be limited, and method parameters are being evaluated. However, in some organizations traditional HPLC continues to be commonplace for routine QC analyses, meaning methods should be designed with transferability in mind. The ability to move analytical methods and product knowledge from R&D through development to manufacturing QC without compromising accuracy, precision, or regulatory compliance is critical, and hinges upon proper column selection.
Sub-2 µm size-exclusion chromatography (SEC) columns with a 4.6 mm ID lose their efficiency benefit with increased system dispersion volume because of their inherently small peak volumes (see Waters application note 720006337 for a systematic in-depth demonstration of LC dispersion volume impact on protein separations for different SEC column configurations).2 Therefore, HPLC applications require a larger diameter column with subsequently larger peak volumes to mitigate the impact of system dispersion volume on separation efficiency. Waters 7.8 mm ID SEC Columns demonstrate consistent chromatographic efficiency across a range of higher system dispersion volumes typical of HPLC instruments,2 albeit with increased time and material consumption compared to the smaller UHPLC column.
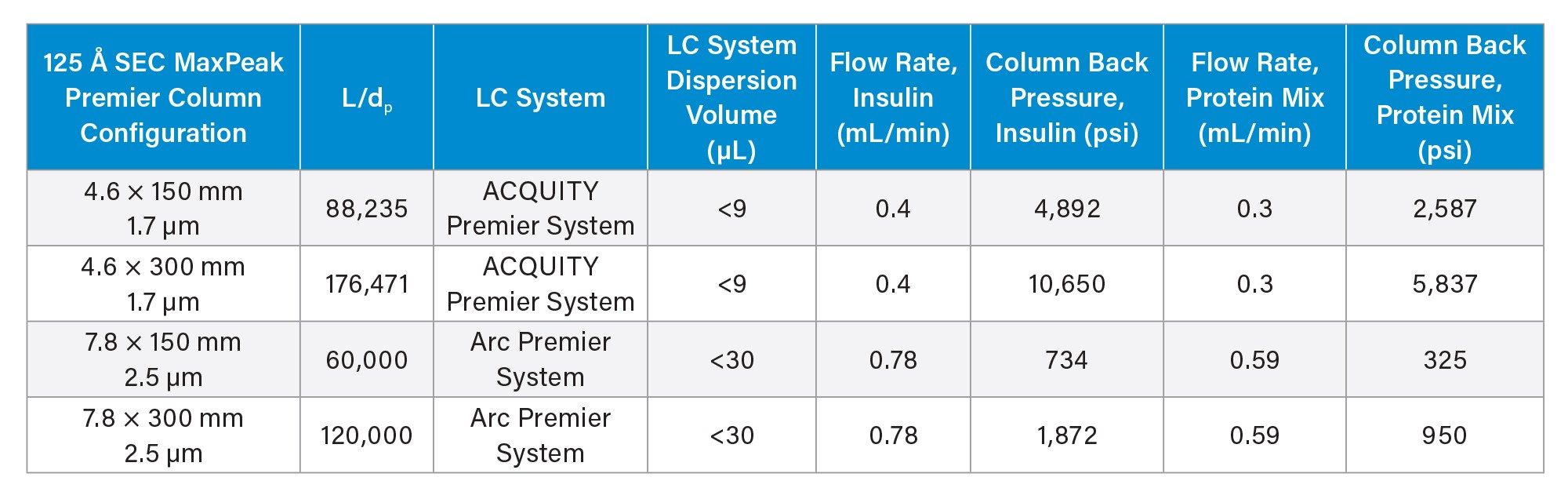
The ability to achieve highly similar chromatographic resolution across two LC systems having different system dispersion volumes has been demonstrated here by appropriately scaling analytical methods from a 4.6 mm ID ACQUITY 125 Å SEC, MaxPeak Premier, 1.7 µm Column to a 7.8 mm ID XBridge™ 125 Å SEC, MaxPeak Premier, 2.5 µm Column. Flow rates were scaled to achieve a constant reduced linear velocity across both the ACQUITY and XBridge Columns, and injection volumes were selected to maintain a consistent sample to column volume ratio.3-4 The USP High Molecular Weight Insulin Standard and the Waters BEH125 SEC Protein Standard Mix (p/n: 186006519) were analyzed as representative peptide and small protein samples.
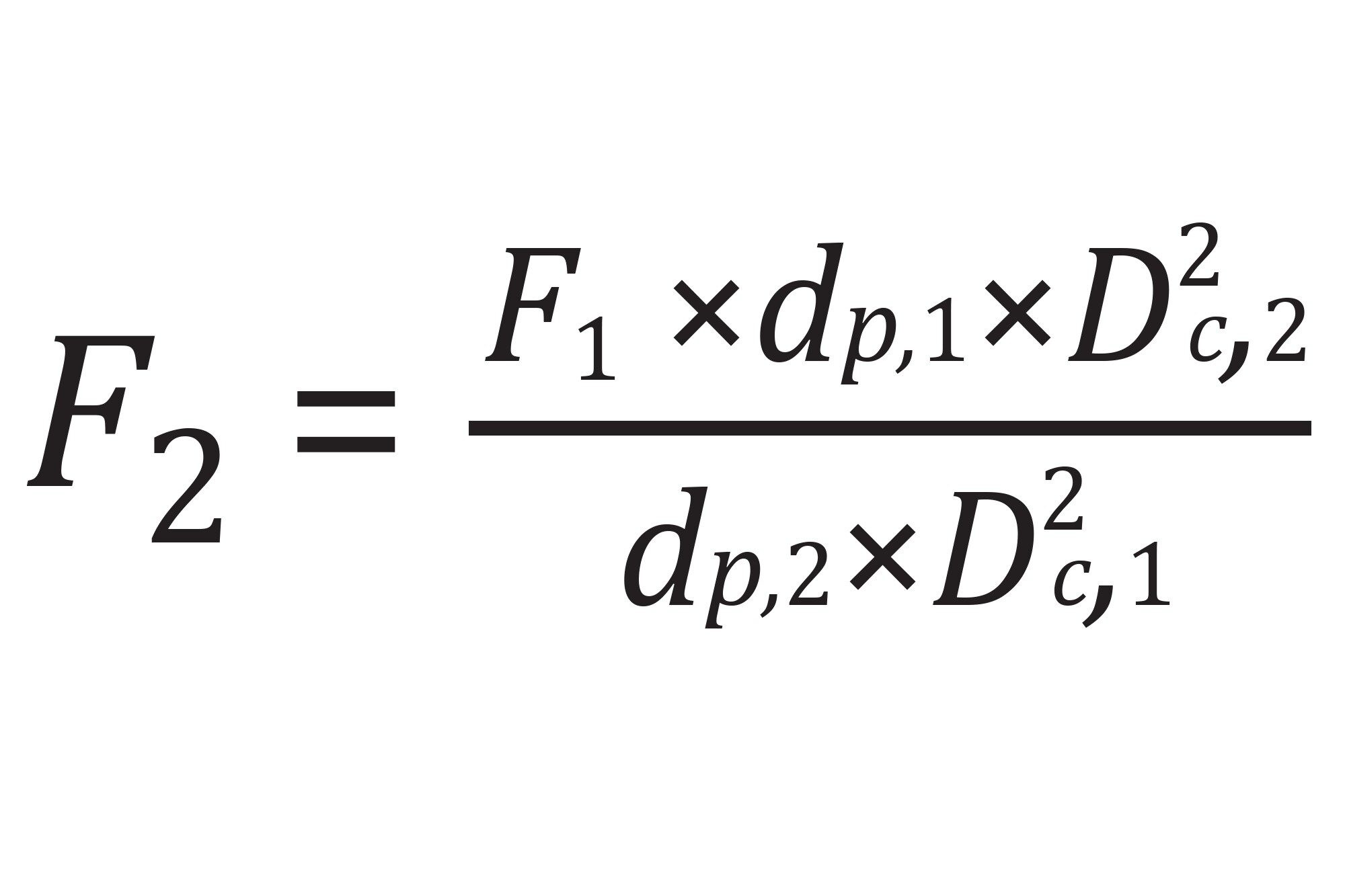
In Equation (1), flow rate (F) for the second column is determined for constant reduced linear velocity by considering the differences in both the column diameter (Dc) and particle diameter (dp). Adjusting flow rate to maintain constant reduced linear velocity is prescribed by USP <621> for chromatography method adjustment.5
SEC analysis of human insulin was performed under denaturing eluent conditions to separate insulin monomer from covalent high molecular weight products (HMWPs) using four different SEC 125 Å, MaxPeak Premier Columns (see Table 1 for summary of LC conditions). Figure 1 shows the insulin chromatograms for the four different column configurations, along with relative peak areas for the HMWPs and the USP monomer half-height (HH) resolutions. The resolution values and HMWPs relative peak areas were comparable for the two 150 mm columns and for the two 300 mm columns, despite a 32% reduction in L/dp ratio from the ACQUITY Column to the XBridge Column. The insulin dimer analysis example highlights the potential of matching ACQUITY Column performance on an LC system with higher dispersion volume by utilizing a 7.8 mm ID Waters column leveraging the same BEH particle technology. However, in some cases, it may be necessary to maintain or increase L/dp to achieve the desired resolution for the transferred method, as seen in the next example.
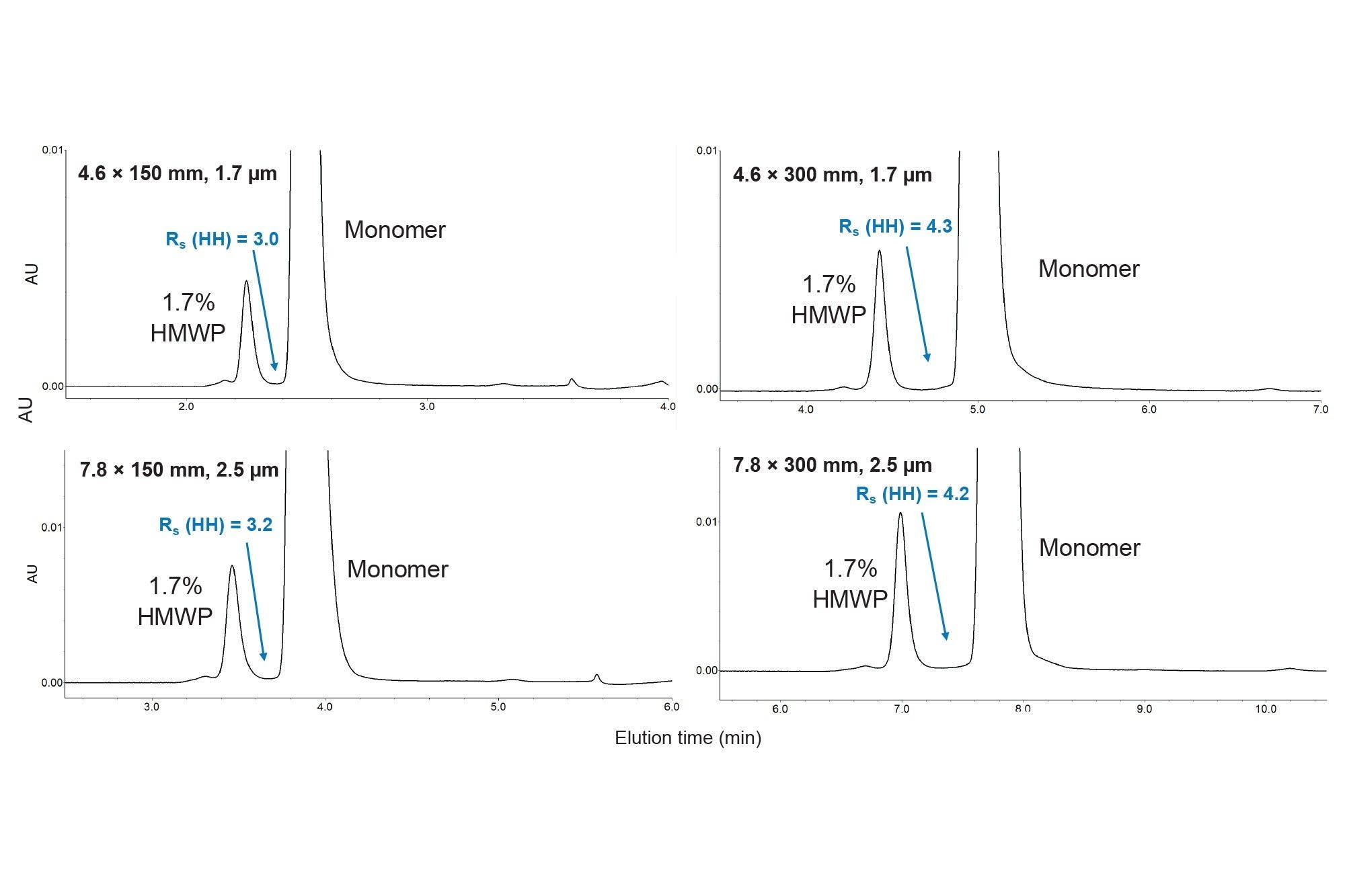
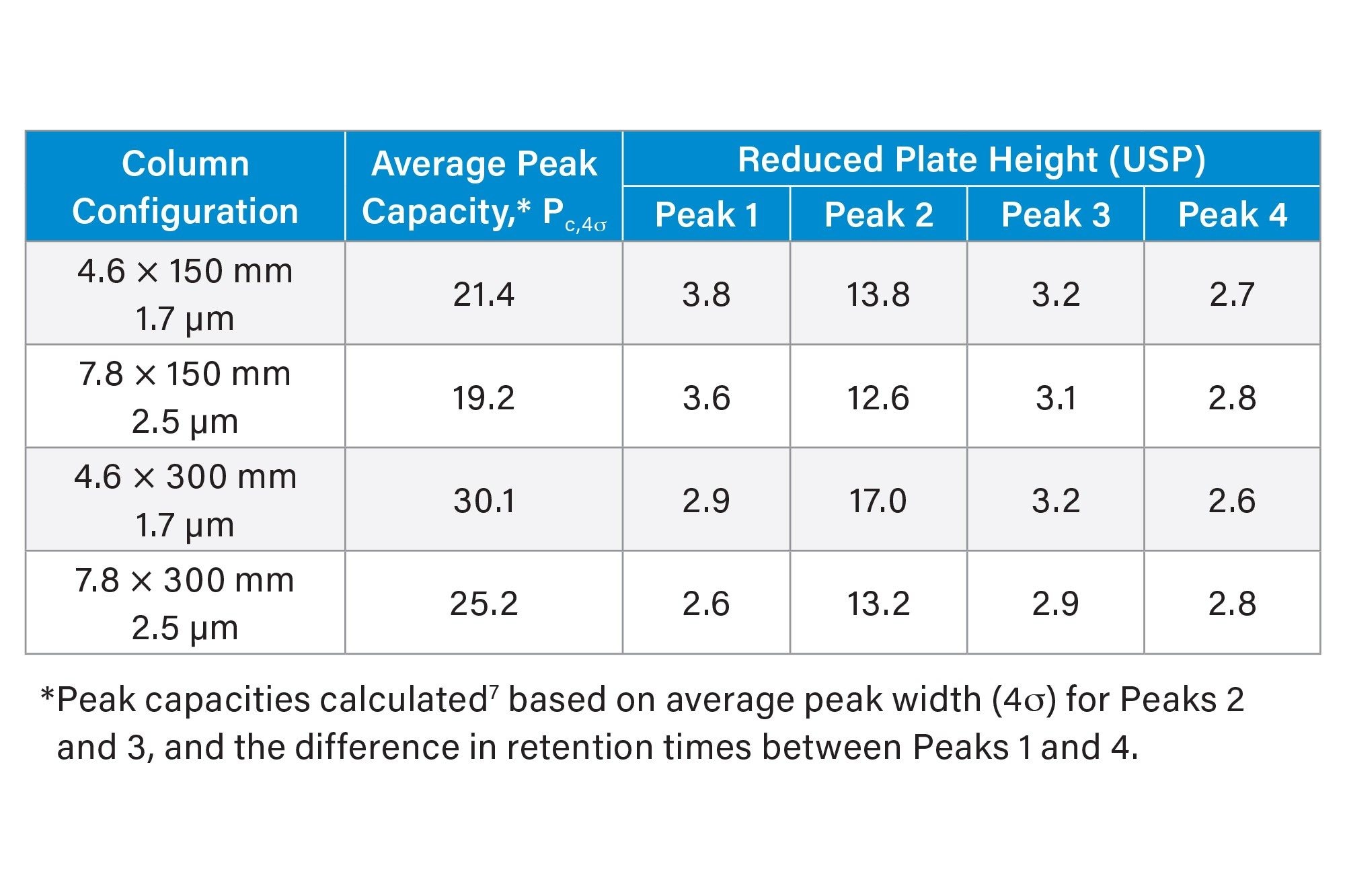
Analysis of Waters BEH125 SEC Protein Standard Mix (p/n: 186006519) under native SEC eluent conditions (Figure 2) showed similar chromatograms for both the ACQUITY and XBridge 125 Å SEC MaxPeak Premier Columns. Average peak capacity and reduced plate height (RPH) values are shown in Table 2 for each of the four columns evaluated. The ACQUITY 125 Å SEC, MaxPeak Premier Columns showed a higher average peak capacity compared to XBridge 125 Å SEC, MaxPeak Premier Columns of the same length. The RPH values, which allow for a comparison of peak efficiency across columns of differing particle sizes, were highly similar for each column length.6
When transferring LC methods, the ratio of column length to particle diameter (L/dp), which directly corresponds to column efficiency, would ideally be held constant. However, it is not always possible to easily match the L/dp value when transferring from UHPLC to HPLC due to limited availability of column configurations. In cases where any reduction in separation efficiency is not acceptable for the method transfer, an increase in L/dp should be considered. USP <621> allows for up to a 50% increase in L/dp for monograph methods.5 For example, a method initially developed using a 4.6 x 150 mm ACQUITY 125 Å SEC, MaxPeak Premier Column (L/dp = 88,235) could be transferred to a 7.8 x 300 mm XBridge 125 Å SEC, MaxPeak Premier Column (L/dp = 120,000), representing a 36% increase in L/dp. Based on the peak capacity results shown in Table 2, the 300 mm XBridge Column had increased chromatographic resolution for the protein mix sample at the same reduced linear velocity. Increasing the linear velocity (i.e., flow rate) for the analysis with the 300 mm XBridge Column will decrease the observed peak capacity. To demonstrate this effect, an additional flow rate of 0.86 mL/min was tested, resulting in an average peak capacity of 23.2, a reduction from the 25.2 value observed at 0.59 mL/min and closer to the average peak capacity of 21.4 achieved with the 4.6 x 150 mm ACQUITY Column (Table 2). When transferring a method to a column with a higher L/dp ratio, the potential exists to increase the flow rate above the value calculated with Equation (1) and still achieve the desired chromatographic resolution.
In this Application Brief, SEC analysis of representative peptide and protein samples with Waters ACQUITY and XBridge 125 Å SEC, MaxPeak Premier Columns demonstrated the ability to transfer methods across different LC systems while maintaining consistent separation efficiency. While the 7.8 mm XBridge 125 Å SEC, MaxPeak Premier, 2.5 µm Columns require larger volumes and longer run times, they provide comparable separations on LC systems with higher dispersion volumes at lower operating pressures.
By incorporating the same QC batch-tested BEH particle chemistry across multiple column configurations and particle sizes, SEC 125 Å, MaxPeak Premier Columns enable analytical method development and QC transferability for size-based separations of peptides and small proteins. Points of note when transferring methods include:
720009011, September 2025