This application note is on multidimensional chromatography, a technique inherently perceived as being difficult to operate and understand. Here we describe how it is possible for inexperienced users to produce quality results in a short amount of time.
In the spring of 2016, after the acceptance of a co-authored research publication between two principal investigators from Boston University School of Medicine and Waters Corporation, a project to expand into a collaborative agreement was submitted to Waters Scientific Steering Committee. The goal of the agreement was to pool resources from both research teams. Five- to eight-month internships at Waters would be offered to graduate students from Boston University’s department of Biomedical Forensics Sciences.
There was overwhelming interest from students. As part of the program, interns received daily one-on-one theoretical and hands-on training in mass spectrometry, 2D and 3D liquid chromatography, and sample preparation techniques. After mastering their laboratory skills, each intern was assigned to a research project that aligned directly with their thesis research for their M.S. degree. Project results were also made available in Waters application notes, peer-reviewed publications, and oral/poster presentations at select conferences.
In the spring of 2018, the project collaboration expanded to include a five-day, intensive, advanced chemistry laboratory class for five students (the first of its kind). After the first day of class, one student opted to intern at Waters that fall. By year-end, with a research project on microcystin analysis in urine by 2D LC-MS/MS nearing completion, the intern was offered a full-time scientist position at Waters for January 2019. In the spring of the same year, the collaboration hosted its second five-day advanced chemistry class with six student interns (Figure 1).

This application note is on multidimensional chromatography, a technique inherently perceived as being difficult to operate and understand. Here we describe how it is possible for inexperienced users to produce quality results in a short amount of time. The interns were challenged with this task and how to create a 2D LC-MS/MS protocol for the analysis of targeted molecules in a biological matrix. Each day had a main objective, and the day began with a one-hour lecture, leaving the remainder of the day for hands-on practice.
In a multi-task environment the students were trained on how to generate maximum results, and how to manage day-time workflow with day and overnight data acquisition. The raw results were tabulated in excel spreadsheets, MS spectrums and LC chromatograms provided in PowerPoint, and all data was made available for publication. This application note reflects the students’ work and interprets the students’ training during the five-day class.
When using LC-MS platforms, most users are confronted with analytical challenges that require very complex sample preparation protocols, thus producing complex extracts. In this case, the number of entities or analytes present in the final extract will largely exceed the separation power (peak capacity) of a single dimension chromatography system. Novel separation approaches, specific detection, and extraction chemistries can help, but those will usually produce limited performance. In recent years, many applications are coupling multiple layers of separation dimensions in the attempt to increase the separation power for the analysis of the complex mixture. Today, the concept of multidimensional chromatography is still perceived as a very difficult technique to master. By overlooking the perception of complexity, multidimensional chromatography simply adds extra components in order to achieve a specific workflow. Entry-level upgrades are 10–20% of the cost of a standard LC-MS/MS system. The return on investment produces an average 10-fold cost reduction in sample preparation protocols, analytical time, consumables, and resources.
The following are the intern’s and student’s thoughts on their experiences with the collaborative program and LC-MS/MS.
Malorie Mella, Boston University School of Medicine, Class of 2017: “My internship had an incredible impact on my career trajectory. The opportunity to build upon my fundamentals in chromatography and mass spectrometry with hands on experience such as setting up instruments, troubleshooting, and doing research for application notes was invaluable. Learning about the advantages and experimenting with 2D LC techniques truly cemented my working knowledge of how LC-MS could be applied in industry. I was also able to ace interviews and gain employment as an analytical chemist at a start-up pharmaceutical company where I single handedly developed several analytical methods for testing drug formulations in development using all the knowledge and techniques I learned during my internship. I am very thankful for my time there and owe my career to it.”
Kayla Benvenuto, Boston University School of Medicine, Class of 2017: “My internship expanded my experience level, skill set and knowledge extensively. From sample preparation to method optimization, I was able to apply what I learned in the classroom and in textbooks hands on. I was given guidance and gained skill sets to be able to work independently. More importantly, I gained essential troubleshooting skills which have become imperative in my current employment position. Multidimensional chromatography was a major contributor to my skill set. Overall, I acquired extensive knowledge of chromatography.”
Brendan Scheitzer, Boston University School of Medicine, Class of 2017: “My internship exposed me to a large volume of hands-on experience. Overall, it was a great boon in understanding, focusing on practical instead of just theoretical, the complexities of chemical analysis using LC-MS/MS. I was paired to a daily one-on-one trainer ready and willing to share his wealth of knowledge with me. The experience I garnered there were unmatched and invaluable to me; it is hard to express just how much I learned from my time working at Waters Corporation.”
Robert Walsh, Boston University School of Medicine, Class of 2018: “My internship was incredibly useful and helpful both from a scientific learning and professional development perspective. On the scientific side, it provided more handson and in-depth experience with LC-MS/MS than could be obtained in any academic environment. It also allowed for me to learn multidimensional chromatography, a newer frontier in LC-MS/MS analyses that helps to improve LC performance and that is relatively simple to learn once you have a working understanding of LC. From a professional perspective, it gave me a taste of what working for private or industrial sector is like. Furthermore, it certainly helped my job applications to have industrial experience on my resume.”
Jacob Samuel, Boston University School of Medicine, Class of 2018: “As someone who was fascinated with instrumental analysis and wanted a deeper and more applicable education than what I had, this internship was perfect for me. Given that my project dealt with multiple classes of compounds, I was given ample practice and a variety of scenarios to learn and troubleshoot in sample preparation, chromatography and mass spectrometry. Additionally, I learned a good deal about innovation, not only in learning about and using multidimensional chromatography, but also pursuing better ways to meet one’s needs. On top of that, having a wide selection of tools available can really open your eyes to what is possible in method development. Ultimately, the industrial internship provided a great environment to learn and explore provided one is willing to put in the effort.”
Beatriz Renner, Boston University School of Medicine, Class of 2019: “The impact that my internship had on my career as a scientist was tantamount to having had industry experience. Once in my post as a Scientist in Waters Scientific Operations group, I was able to start hands on work immediately. All the skills I received during my internship have not only been helpful but necessary to perform my current job duties as a scientist.”
Devyani Bhandari, Boston University School of Medicine, Class of 2019: “My experience as an intern at Waters Corporation has been very enlightening. This internship has not only advanced my scientific knowledge but also prepared me to fit right into any industrial setting. It also has made me into a problem solving and self-motivated individual. The hands-on training provided me with hands on skills that are in demand across many fields like pharmaceuticals, environmental, forensics, and many more. I believe this internship is an asset to the BMFS program and has helped me standout from my competitors in terms of skills, knowledge, and experience.”
Ketki Bagwe, Boston University School of Medicine, Class of 2019: “As I came from a background of biology, I had no experience with LC-MS/MS apart from some theoretical basics. Despite this, within a couple of weeks, I was able to handle the LC-MS instrument independently. The learning curve was steep, but it was an enjoyable and educational process. The internship is very hands on, which gives you the necessary skills required for a lab-based job and helps polish your knowledge of chemistry. I was given the opportunity to take ownership of my project, and this along with being able to work with and troubleshoot an LC-MS instrument on your own gives one a confidence as well as a skill boost. As this lab specializes in multi-dimensional liquid chromatography, it is a great place to learn about cutting-edge research projects. It proved to be a great learning experience and in addition gave me an exposure to industry as well as corporate culture.”
Olivia George, Boston University School of Medicine, Class of 2020: “In attending the Advance Chemistry course at Waters Corporation, I was able to get a taste of the kinds of technologies used in the real world and have hands-on experience with cutting-edge instrumentation. Going through the process of understanding sample preparation, chromatography, and mass spectrometry gave me valuable insight and pushed me to want to take an internship at Waters. The work and life skills, such as time management and being able to work independently, learned here will be of paramount value in future job searches. These skills will allow me to be far ahead of my competitors in the job market.”
Miranda Shaine, Boston University School of Medicine, Class of 2020: “Taking the Advanced Chemistry class at Waters Corporation was one of the most educational and eye-opening experiences I have had. The privilege of working hands on with the instrumentation that is used universally in many scientific fields taught me how to apply the knowledge I have learned directly to practical uses. The expertise and guidance of the teacher inspired me to take an internship at Waters Corporation, providing a one-on-one, hands on opportunity to learn the ins and outs of the application. I am confident that once I complete the internship, I will have the knowledge and ability to be a strong candidate for other professional opportunities.”
Synthetic cannabinoids belong to a class of drugs known as novel psychoactive substances. Such substances are manufactured to produce similar effects to illicit drugs but are currently unregulated. With changing confirmations and the rapid evolution of compounds, identification and quantification of these compounds can be difficult with routine screening and confirmatory tests. Developing a method for the rapid and sensitive screening and quantification of these compounds is useful in identifying the presence of these increasingly abused compounds.
Furthermore, antibiotics are used in the treatment of bacterial infections. In administering antibiotics, therapeutic monitoring of dosage is important as the incorrect dose can lead to bacterial resistance or excessive elimination of naturally endogenous beneficial bacteria.
This research sought to develop a method for the rapid and sensitive screening and quantification of several compound classes using two-dimensional, ultra-performance liquid chromatography (UPLC) with tandem mass spectrometry (MS-MS). Traditional HPLC columns are 4.6 μm × 100 mm and packed with a synthetic sorbent. Silica particle beads are often used to obtain unchanged normal-phase chromatography. A ligand, typically C18, is added to create a non-polar stationary phase for reversed-phase chromatography. In UPLC there is higher pressure, however the flow rate drops compared to HPLC. A guard column is introduced to increase the life of the column and filter out the debris or contaminants.
Additionally, various matrices can complicate extraction processes, which makes it difficult to detect or quantify their analytes due to matrix effects. Many laboratories are using sample preparation techniques that include solvent evaporation followed by reconstitution. These steps are not only time-consuming, but also have the potential to lose trace amounts of the desired compounds. Two-dimensional liquid chromatography (2D-LC) allows for robust and reproducible methods that eliminate steps for evaporation and reconstitution. The elimination of these steps decreases sample preparation time without losing the quality of the results.
The process began with optimizing a Multiple Reaction Monitoring (MRM) transition for several target analytes divided into two classes (Figures 2 and 3). Two MRM transitions for each compound were recorded for qualitative and quantitative purposes by selecting the most intense signals for the parent ion. MRM transition data was obtained for each drug class at pH 3, 7, and 10 over a range of different Collision Induced Dissociation (CID) energies. This enabled the 2D-LC instrument to recognize the mass-to-charge ratios of interest in the specific method (Table 1).
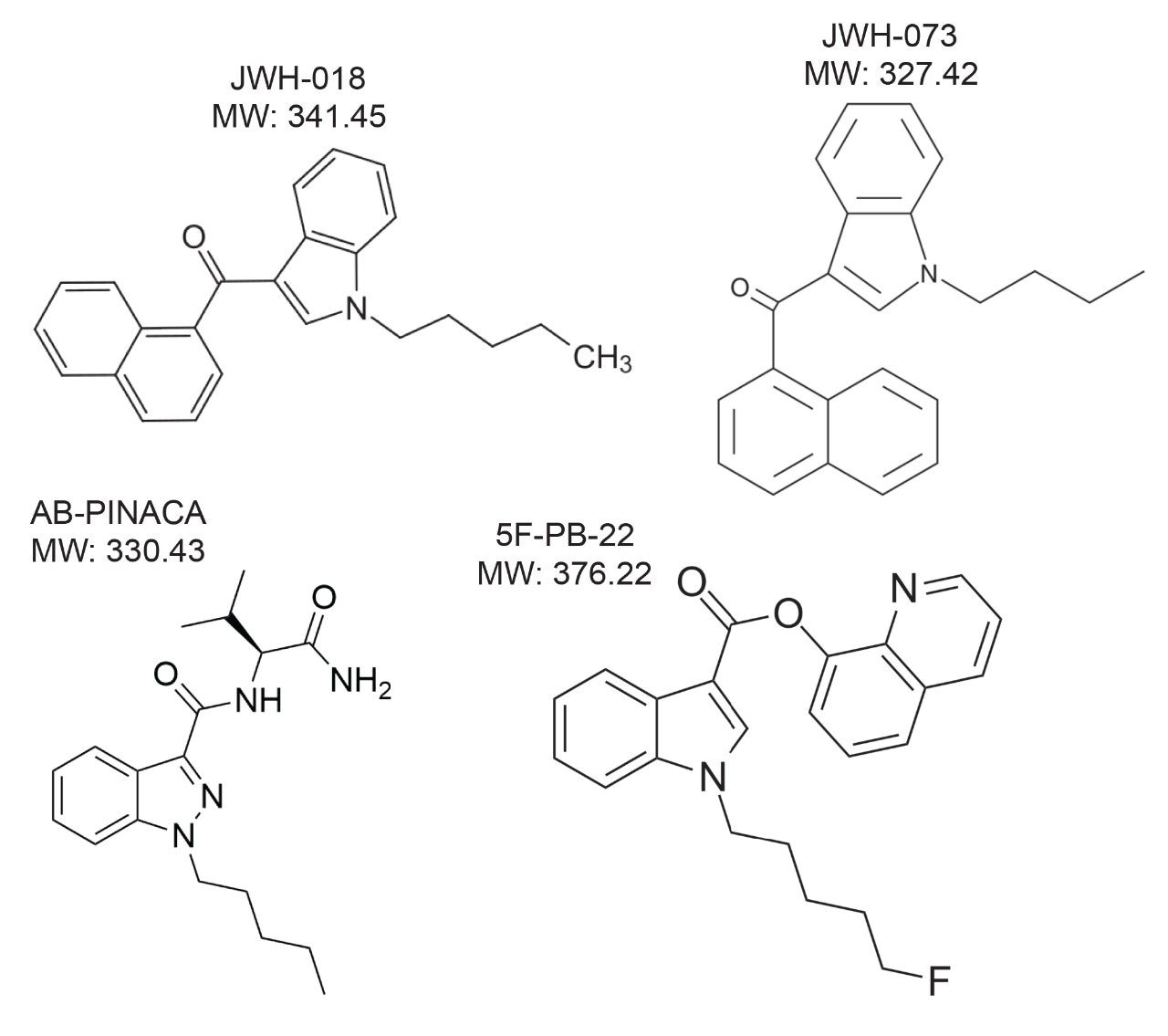
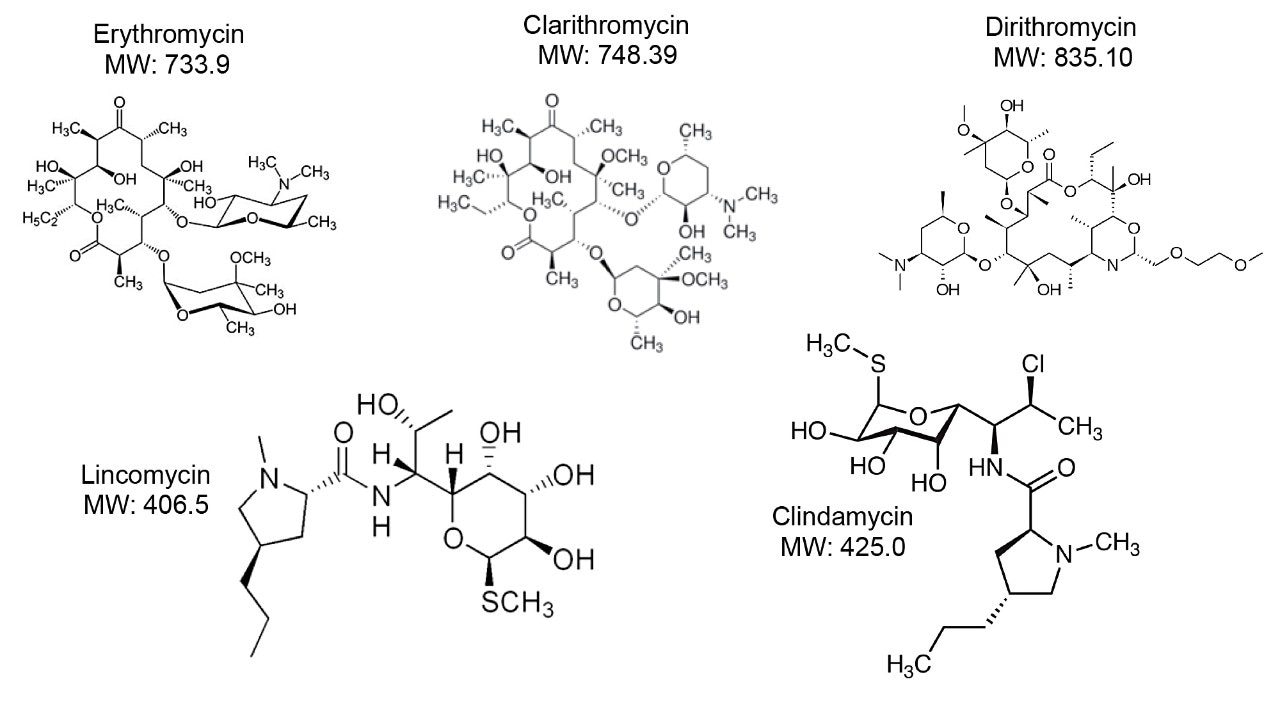
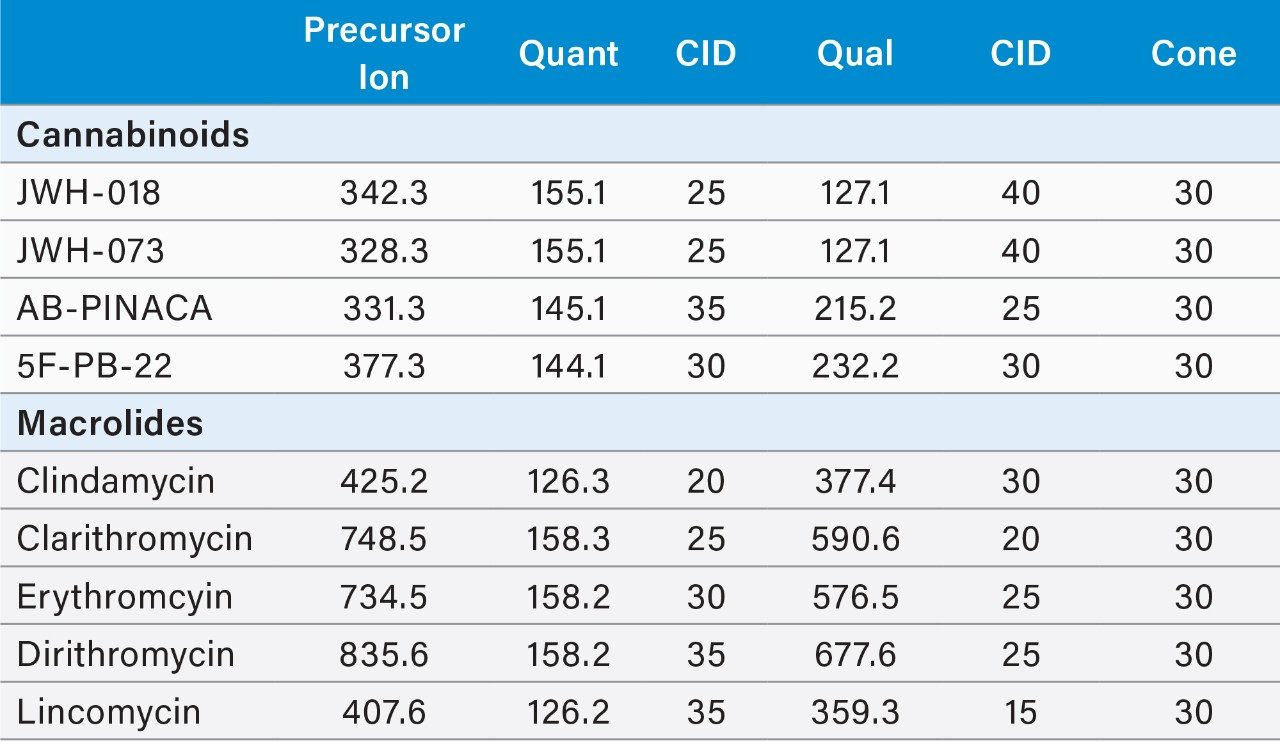
Upon selection of the analyte’s MRM, the next phase was chromatographic condition optimization for each target analyte. Due to the wide range of chemical compositions and polarities of the target analytes, parameters must be established before analysis. The chromatographic conditions were tested on XBridge C18 and Oasis HLB trapping chemistries and BEH C18 separation chemistries. The HLB trap differs from the C18 trap because a polymer is used instead of silica. The loading mobile phase (low pH, high pH, and neutral pH) and eluting mobile phases (MeOH + 0.5% ammonium hydroxide and ACN + 0.5% ammonium hydroxide) were also optimized. The best method for each drug class was selected based on the maximum signal intensity and the ability to display the best Gaussian chromatography peak shape for all the compounds within their respective drug classes.
After selection of the best chromatographic method at the ideal pH, varying solutions (10 ppb in water, methanol, and acetonitrile) were tested to determine the best sample preparation of each compound class to elute for solid-phase extraction (SPE). A seven-point calibration curve was evaluated with concentrations of 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 ng/mL of each analyte for macrolides, with a resultant five-point calibration curve (0.5, 1.0, 2.0, 5.0, 10.0 ng/mL) being utilized for cannabinoids quantification. SPE was performed on concentrations 0.1, 1.0, and 10.0 ng/mL of each class in water and urine samples for comparison to the calibration curve. The SPE column was conditioned with 2 mL methanol, 2 mL water. Then 2 mL of the spiked sample was loaded. The column was then washed with 5% methanol solution and target analytes were eluted with 2 mL of low pH 3 or high pH 10 solvents, including acetonitrile and/or methanol. The extracted solutions and two methanol blanks were analyzed on instrumentation for quantitation. It should be noted that the 2D-UPLC-MS/MS technique does not require the analyst to evaporate the solution to dryness and reconstitute; thereby, decreasing the work time for this method.
|
Loading conditions |
|
|---|---|
|
Column: |
Oasis HLB, 20 μm–40 mg (3.9 × 5 mm) |
|
Loading: |
MilliQ water (pH 7) |
|
Flow rate: |
2 mL/min |
|
At-column dilution: |
5% (0.1 mL/min loading pump, 2 mL/min diluting pump) |
|
UPLC |
|
|---|---|
|
System: |
ACQUITY UPLC with 2D Technology configured for trap and elute with At-column dilution |
|
Runtime: |
10 min |
|
Columns: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 × 50 mm (p/n: 186002350); Oasis HLB Direct Connect HP, 20 μm, 2.1 mm × 30 mm, (p/n: 186005231) |
|
Column temp.: |
60 °C |
|
Mobile phase A: |
Water + 0.5% ammonium hydroxide |
|
Mobile phase B: |
Acetonitrile + 0.5% ammonium hydroxide |
|
Elution: |
5-min linear gradient from 5% (B) to 95% (B) |
|
Flow rate: |
0.500 mL/min (elution pump) |
|
Injection volume: |
50 μL |
|
Injection rate: |
250 μL/min |
|
System: |
Xevo TQ-S |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
2.5 kV |
|
Cone voltage: |
30.0 V |
|
Source offset: |
30.0 V |
|
Desolvation temp.: |
30 °C |
|
Desolvation gas: |
650 L/hr |
|
Cone gas: |
50 L/hr |
The target analytes for this application note were separated into two classes: synthetic cannabinoids and macrolide antibiotics.
Each class contained up to five target analytes sharing a common chemical backbone. The workflow began by creating 1.0 mg/mL
stock solution for each target analyte in either methanol or acetonitrile. From the stock concentration, an infusion solution at
1.0 μg/mL in 50/50 methanol/water was made at three different pHs; acidic (pH 3), neutral (pH 7), and basic (pH 10). Each target
analyte was infused at 10 μL/min under full scan ESI positive, to highlight which pH gave the highest intensity. By comparing
the full scan spectra at various pHs, the task at hand was to identify in which state (e.g., [M]+, [M+H]+, [M-H2O]+, [M+Na]+, etc.)
a target analyte will be present in chromatography conditions (Figures 4 and 5).
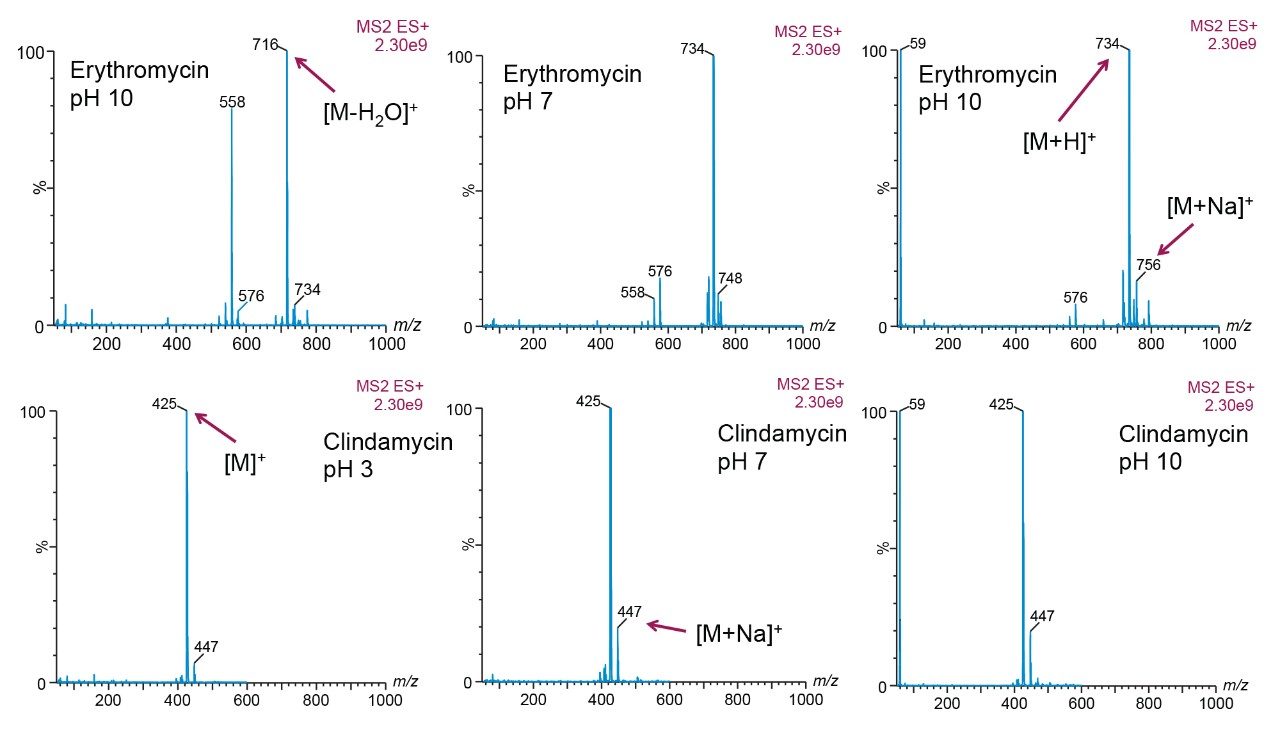
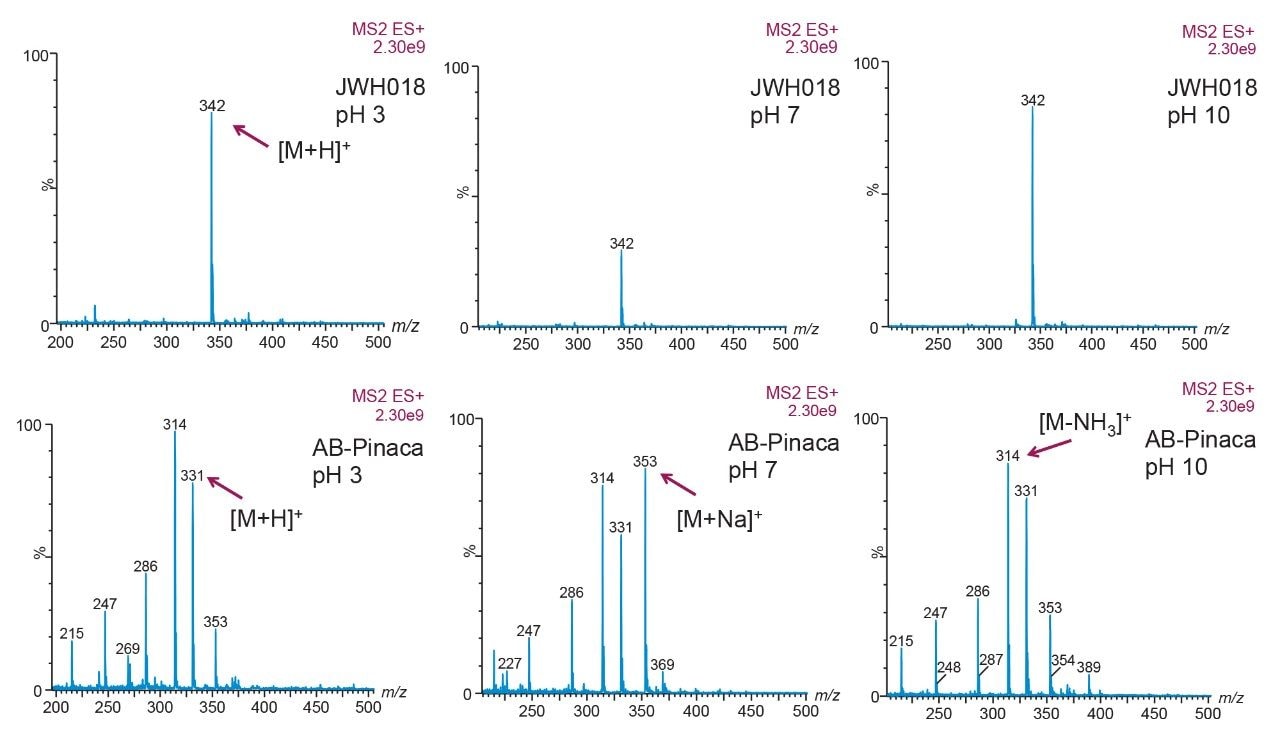
Adducts were observed on the spectra at times, suggesting that another entity connected to the molecule of interest thus increasing the weight of the target analyte. Often, a sodium atom could be attached, or the loss of a water molecule was observed. While this does not diminish the data, it is still important to recognize if it appears in the data.
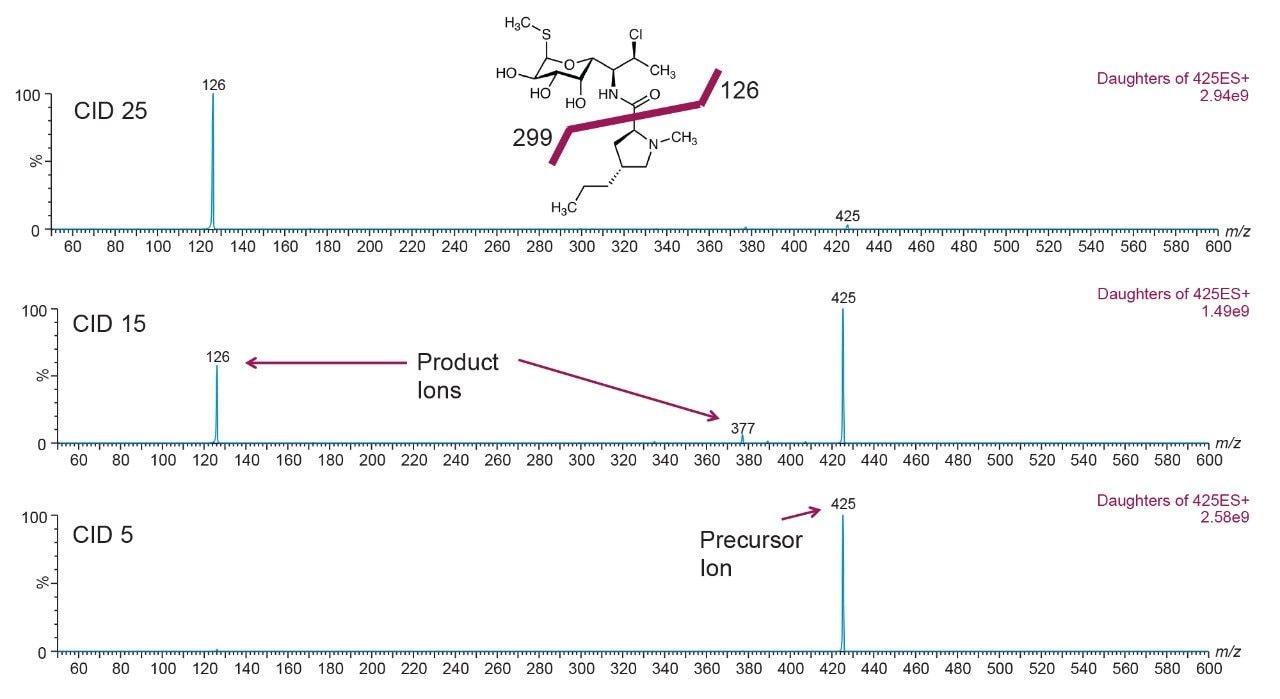
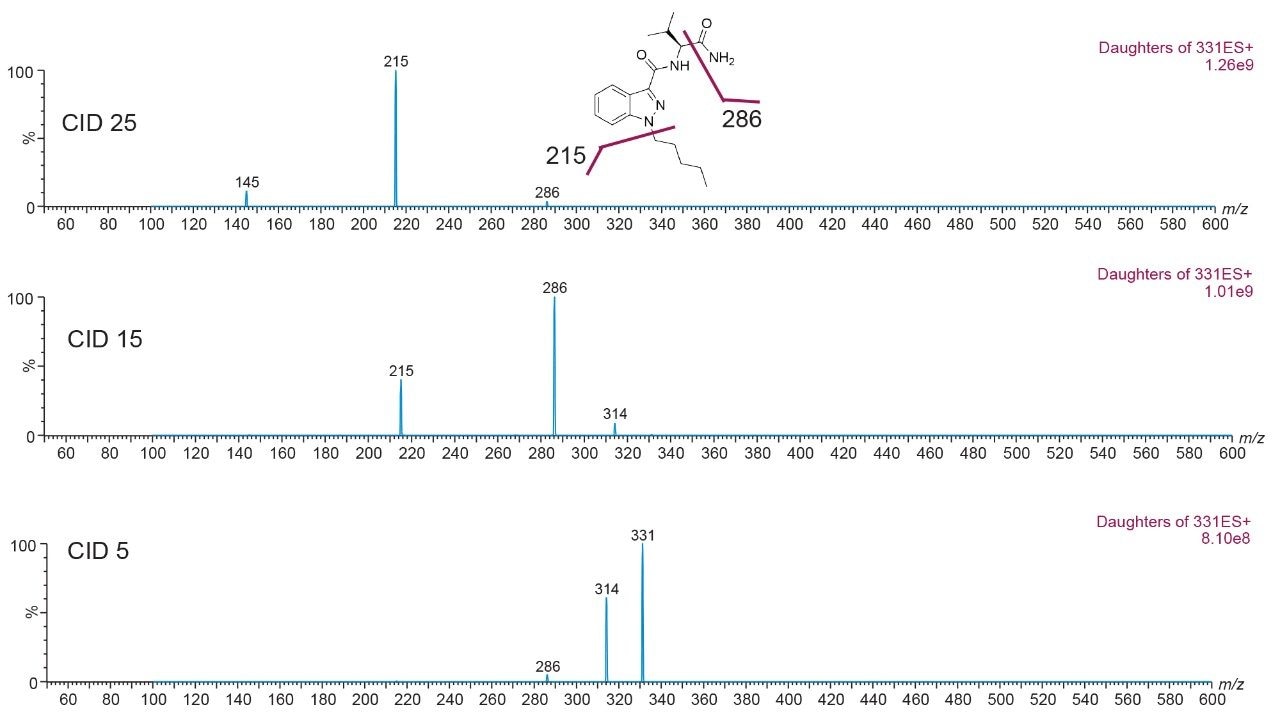
The next phase was to optimize the fragmentation of the precursor ion into product ions by increasing the collision energy. Figure 6 shows a full-scan spectrum (bottom) for clindamycin (m/z 425) and two product ion spectrums at CID 10 and CID 25, while Figure 7 show a similar product ion spectrum for AB-Pinaca (m/z 331).
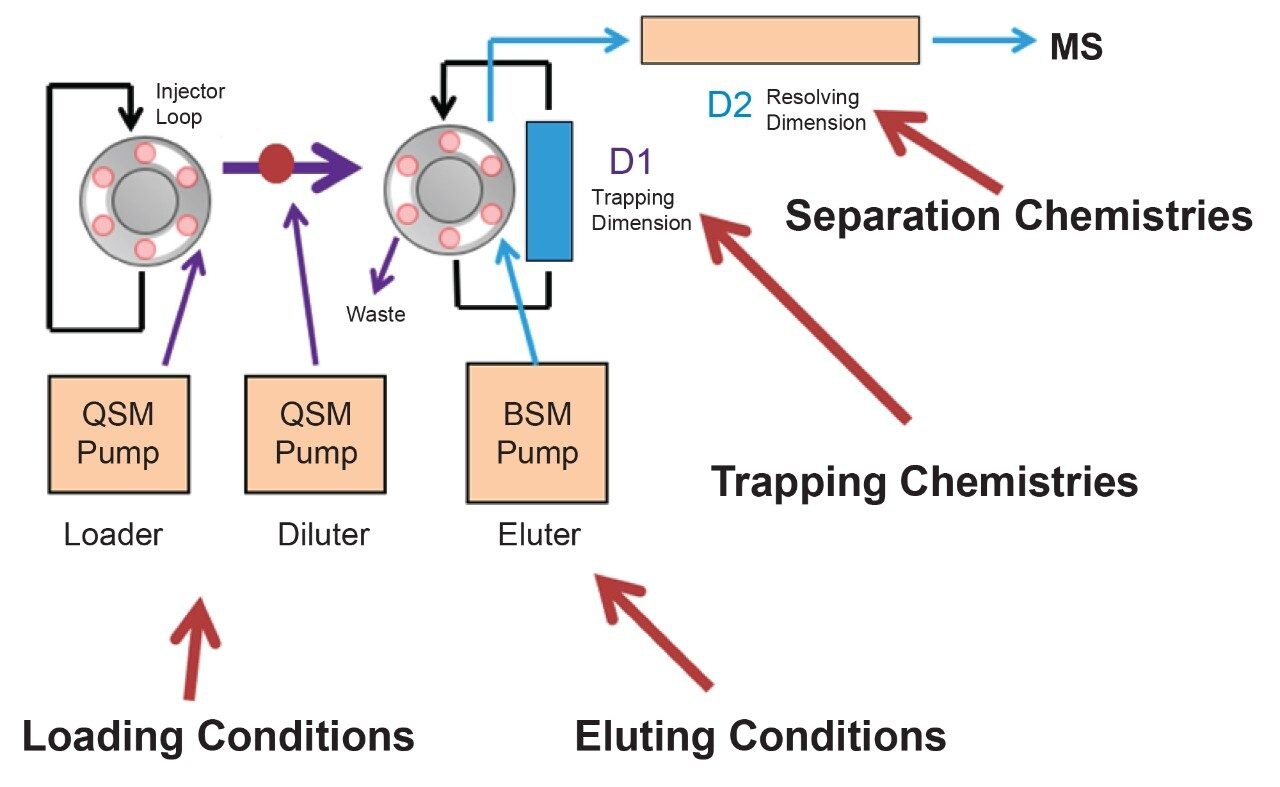
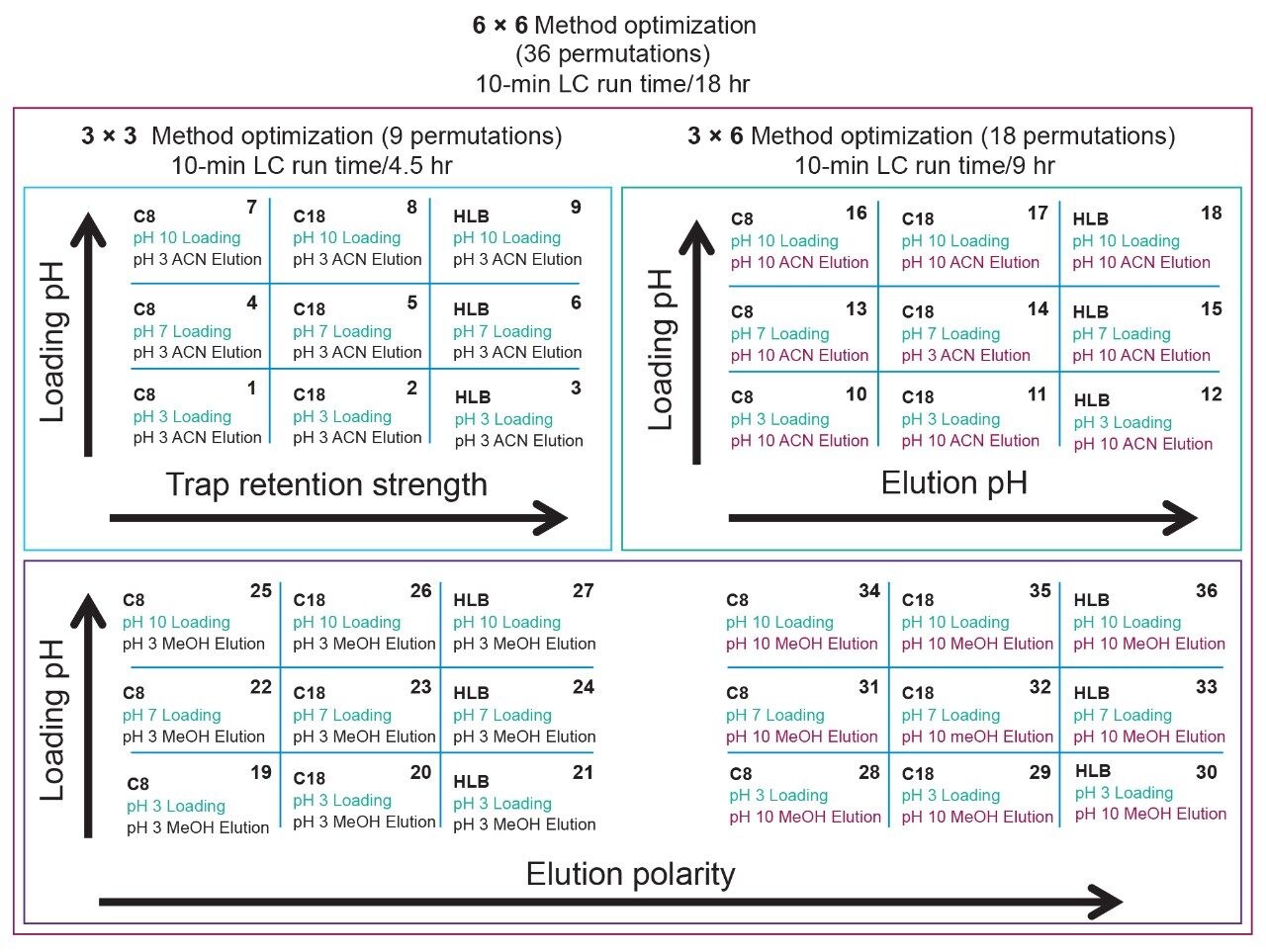
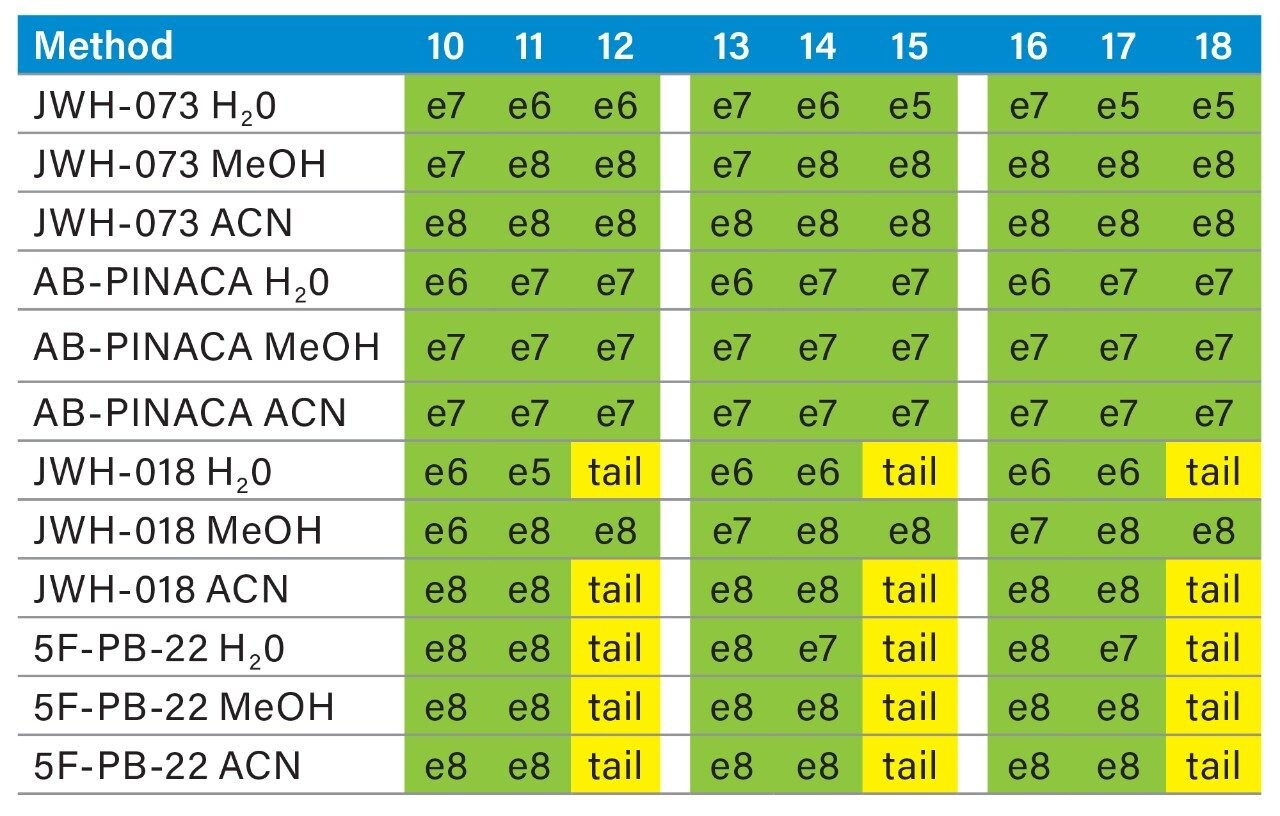
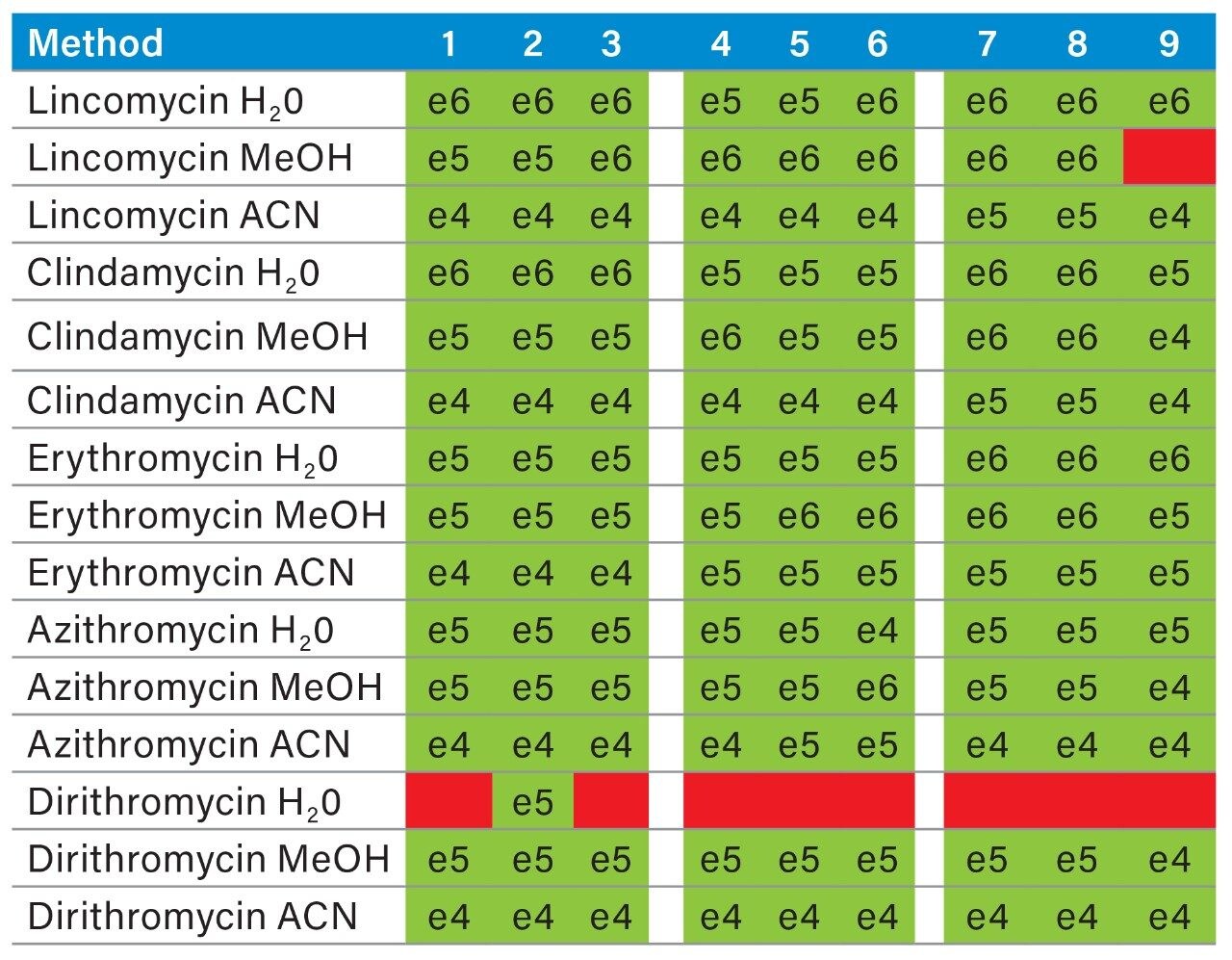
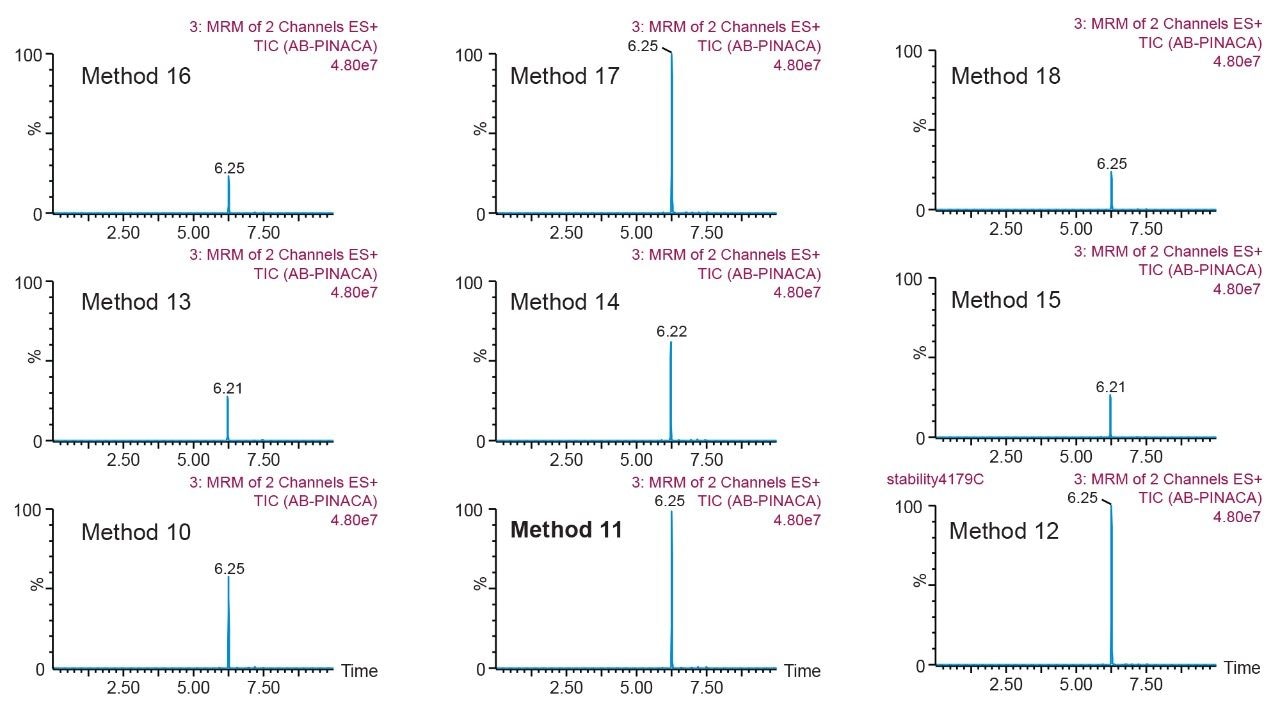
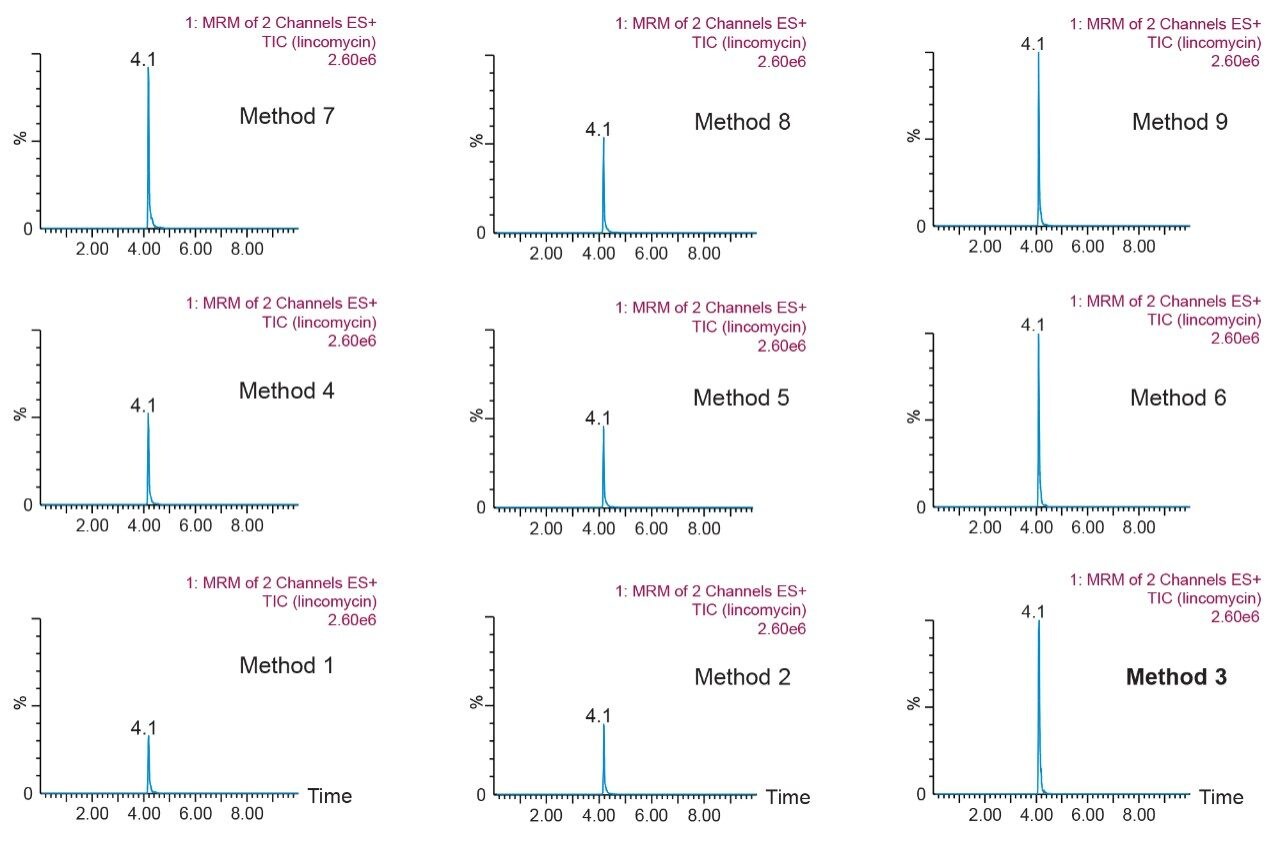
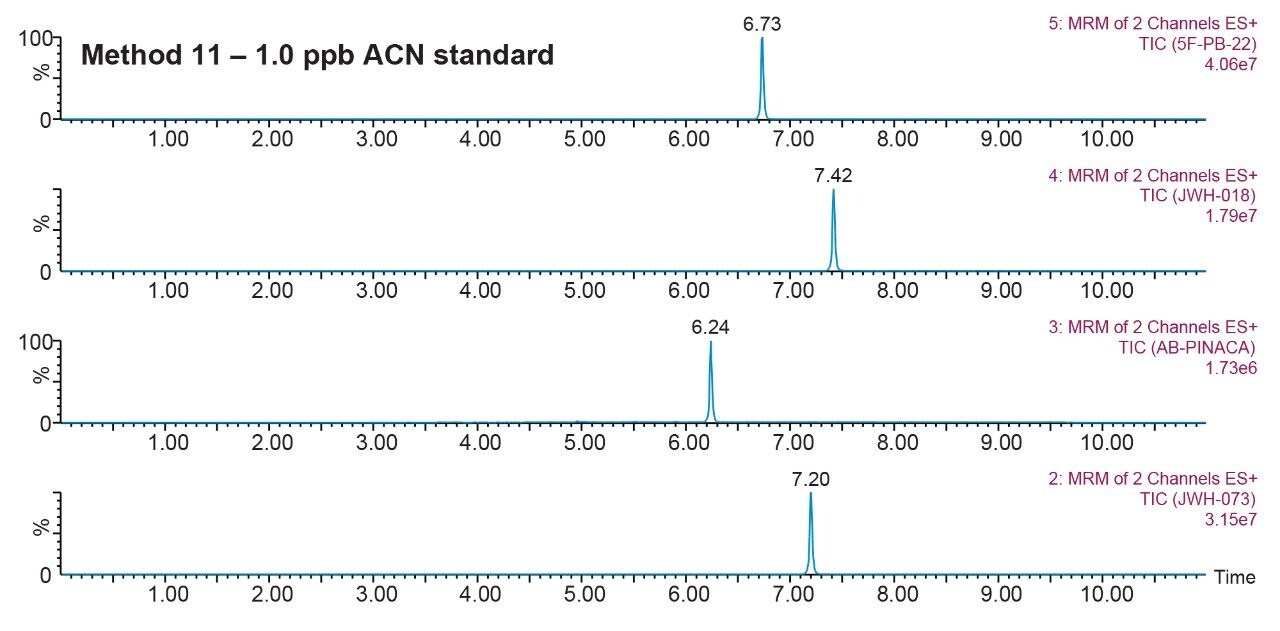
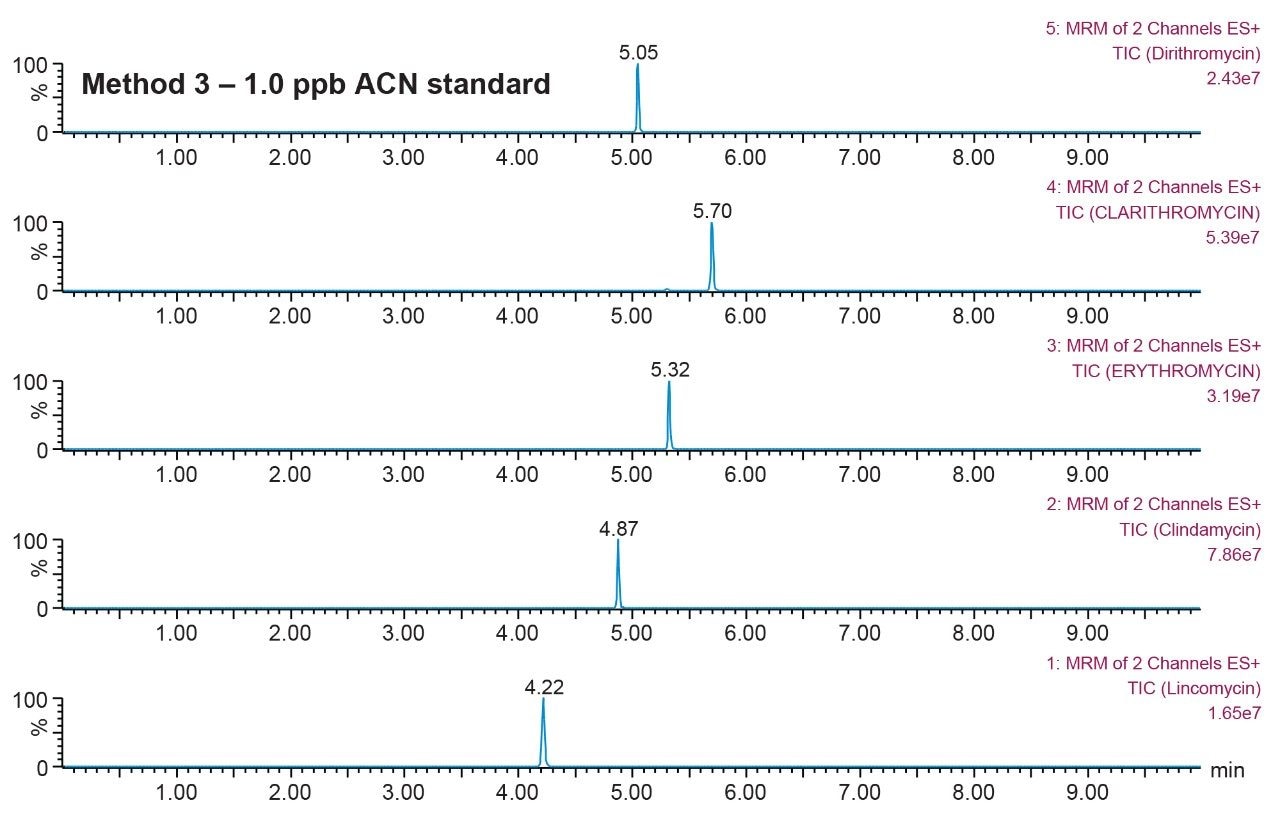
Prior to running urine samples, the 2D-UPLC-MS/MS conditions were optimized. The 2D LC set-up consisted of three pumps (loader, diluter, and eluter), and two columns (trap column with 10-μm particle sizes and an analytical column with 1.7 μm particle size). Two quaternary pumps were utilized for loading and diluting, and one binary pump was for eluting. The loader pump flow rate was set at 0.1 mL/min to carry samples from the autosampler into the 50 μL mixer. The diluter pump had a flow rate of 2 mL/min to reduce the organic content of samples (20:1 dilution ratio). Samples were retained in the trap column, then eluted and separated by the analytical column and eluter pump (Figure 8). The loading, trapping, and elution conditions of different 2D-LC methods were evaluated to determine which would best analyze, detect, and quantitate each target analyte. With the loading and eluting step, different solvents (methanol or acetonitrile) and pH levels (pH 3, 7, or 10) were considered. When trapping, different columns (i.e., C18, C8, or HLB) can affect the elution of the compounds based on their affinity to the column’s functional groups. In total, up to 36 permutations can be selected (Figure 9). But, due to time constraint, quadrant two (acetonitrile elution at high pH) was used for the cannabinoids, and quadrant one (acetonitrile elution at low pH) for the macrolides (Tables 2 and 3). Tables 2 and 3 are color coded to show the different chromatographic evaluation results for the two different compound classes. Green boxes indicate Gaussian peaks in the chromatogram and the intensity of the corresponding signals. Yellow boxes represent any abnormal peak shapes such as leading, tailing, shouldering, or split peaks. Red boxes depict unacceptable chromatograms resulting from noise, elevated baseline, or poor sample retention. For the synthetic cannabinoids, the best was method 11 (Figure 10) which used a C18 trap and analytical column with an elution pH of 10. For the macrolide antibiotics, the optimal method was three which utilized HLB columns that had a pH 3 elution (Figure 11). Each method was chosen because they resulted in Gaussian peaks with high signal intensity for all compound classes (Figures 12 and 13).
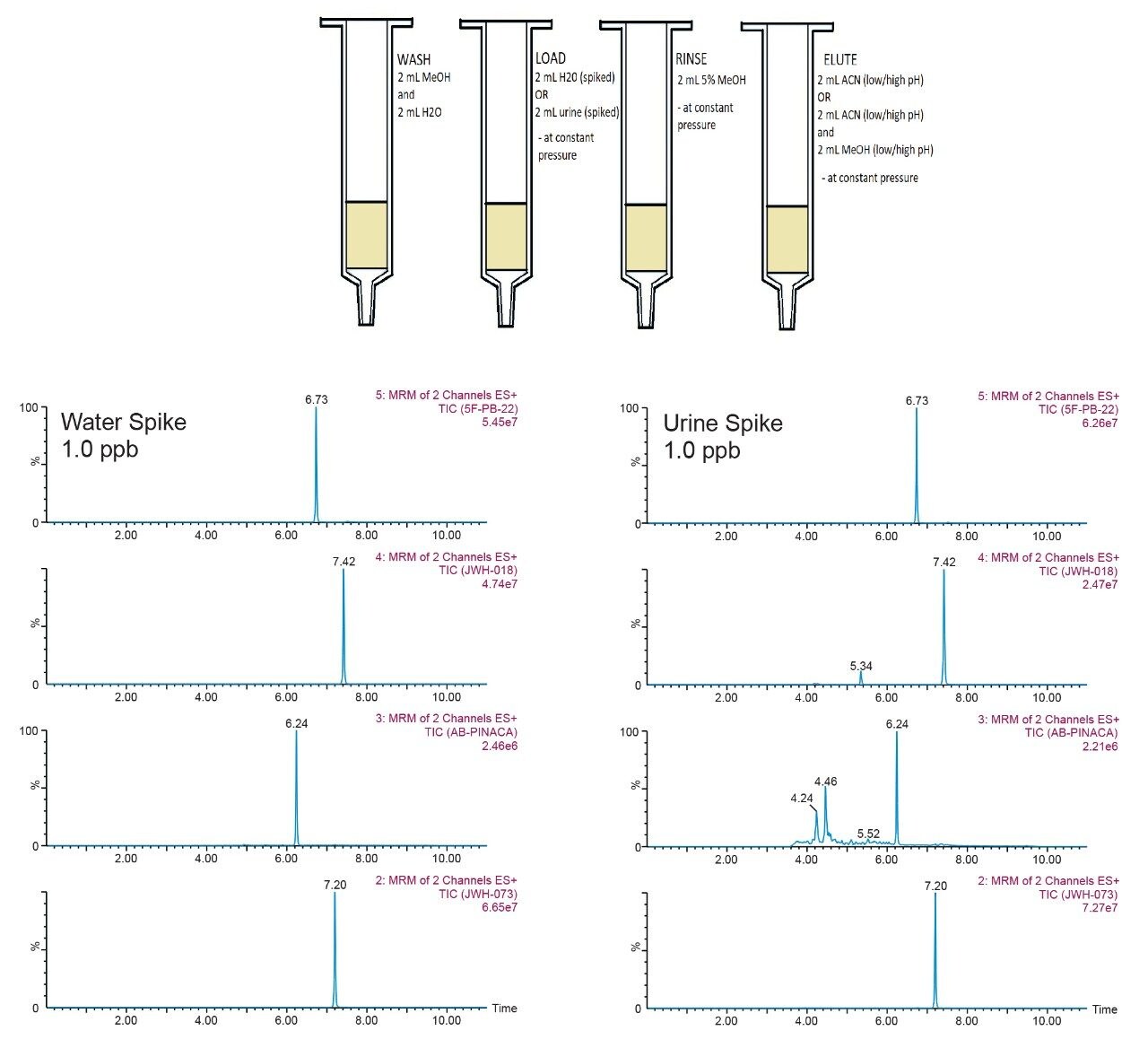
Solid-phase extraction (SPE) was optimized by loading various solutions through a column packed with a sorbent material. Compounds adsorb to the stationary phase based on their polarities and chemical interactions with the stationary phase and mobile phase. The protocol in Figure 14 showcases a four-step process: (i) condition, (ii) load, (iii) wash, and (iv) elution. Each step must be done in the correct sequence. A typical extraction protocol for a 1D LC method will require two additional steps, evaporation-to-dryness with nitrogen stream and reconstitution with compatible initial mobile-phase conditions. Those steps are necessary and very time consuming. However, since a 2D LC approach was utilized for this work, 100% organic solvents can be loaded without any risk of breakthrough. Both the evaporation-to-dryness and reconstitution steps are simply eliminated from the protocol.
The optimization continued with an unextracted seven-point calibration curve from 0.1 ng/mL to 10.0 ng/mL of each class in methanol standards (Table 4). Each concentration was injected as a triplicate injection. The results showed excellent linearity (r2 value of 0.995 and higher) for all analytes over the three orders concentration range. The results showed good linearity for both the cannabinoids and macrolides. The 0.1, 1.0, and 10 ng/mL standards show a clear 10x signal increase, thus confirming that the calibration is well within the linear dynamic range of the ESI source. With higher concentrations, response signals will plateau due to multiplier saturation. With lower concentrations, it is a common trend to see response signals producing a similar flat profile. For the cannabinoids, JWH-018, JWH-073, and 5F-PB-22 are still giving an intense signal suggesting that the detection limit could be pushed to another order of magnitude and reach 0.01 ng/mL. AB-Pinaca shows a weak response at 0.1 ng/mL. The macrolides also show the same intense signal at 0.1 ng/mL concentration and can have a lower detection limit at 0.01 ng/mL.
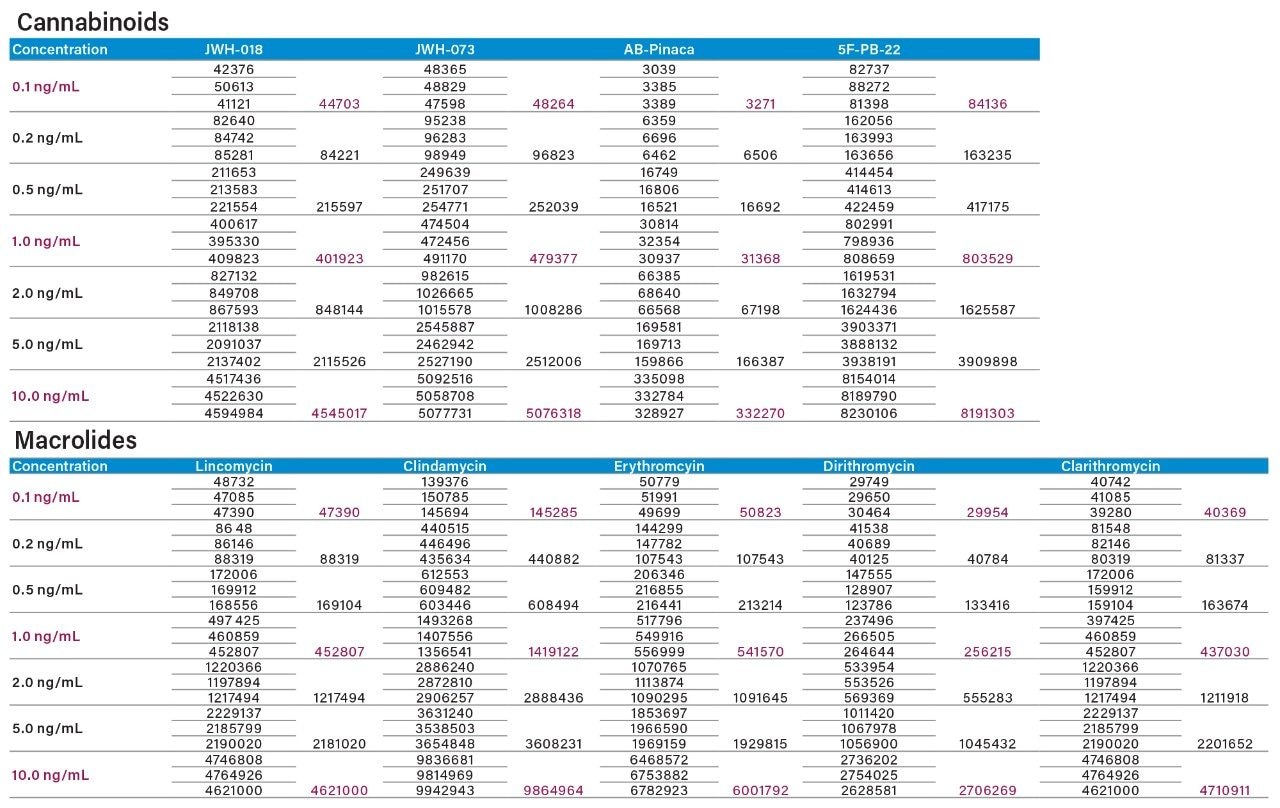
Three calibration points (0.1, 1.0, and 10 ng/mL) were spiked in water and urine samples, representing an extracted standard curve and matrix-match-extracted curve, respectively (Tables 5 and 6). Two elution conditions were evaluated for the extraction protocol. Both the aqueous and urine spiked samples were eluted with 100% methanol at pH 3 (2% formic acid) and 100% methanol at pH 10 (2% ammonium hydroxide). The rational for the different pH values was to evaluate which elution condition (neutral or ionized) would produce the highest recoveries. The aqueous spike was used to calculate the extraction protocol recoveries against an un-extracted standard, without any sample matrix effects. The urine spike recoveries were calculated against an extracted standard giving a measurement of matrix effects in relation to the overall performance of the extraction protocol for intermediate complex samples. In Tables 5 and 6, the un-extracted standard for 0.1, 1.0, and 10.0 ng/mL values from Table 4 are listed in the first column for each targeted analyte. The next set of values are the area counts for the methanol high pH and low pH for the aqueous spikes. The calculated recovery values showed a consistent >75% range for all analytes at pH 10, except for erythromycin at 50%. Because all analytes share a common basic functionality, the results suggest that most of the analytes were eluted under ionized conditions.
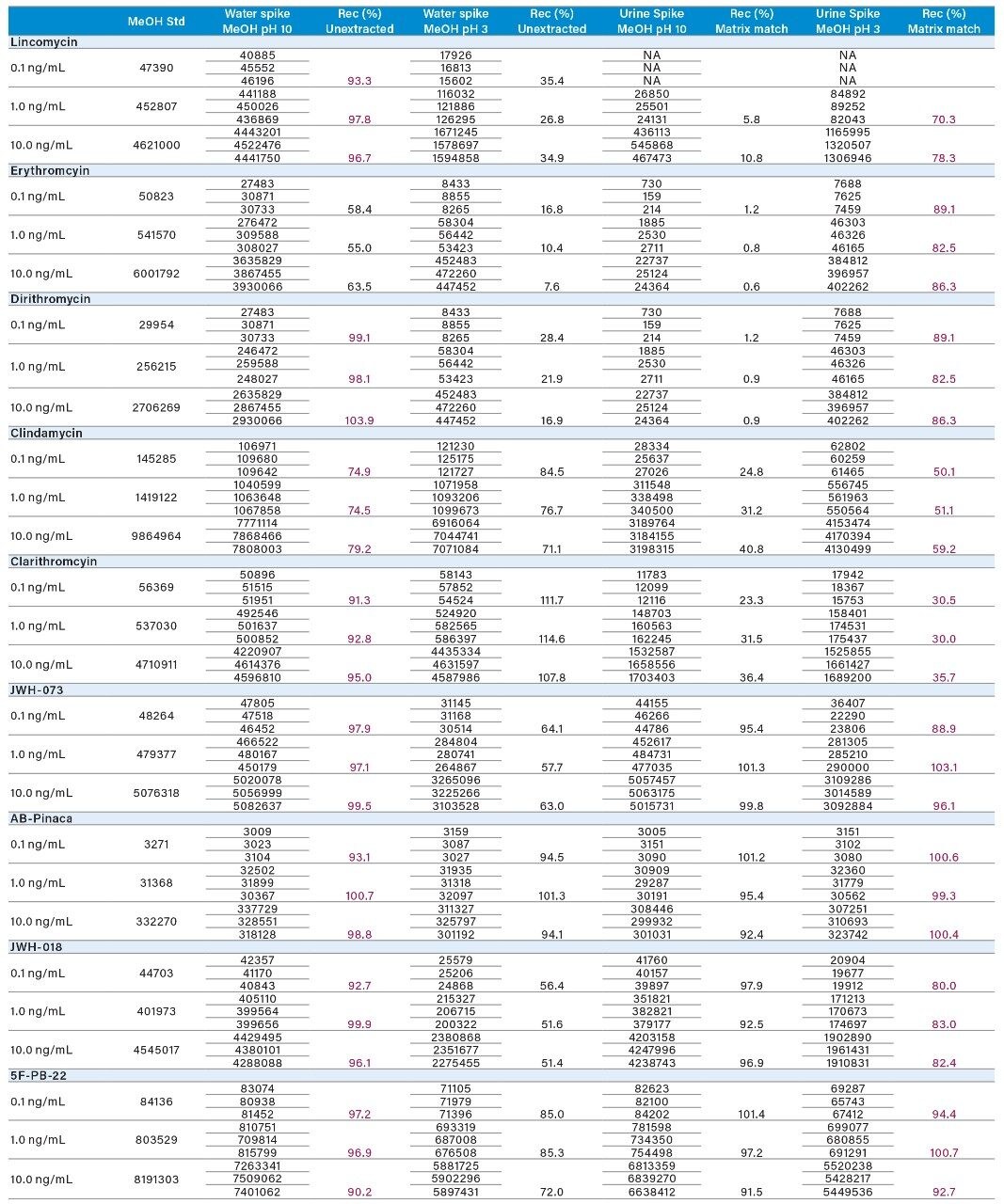
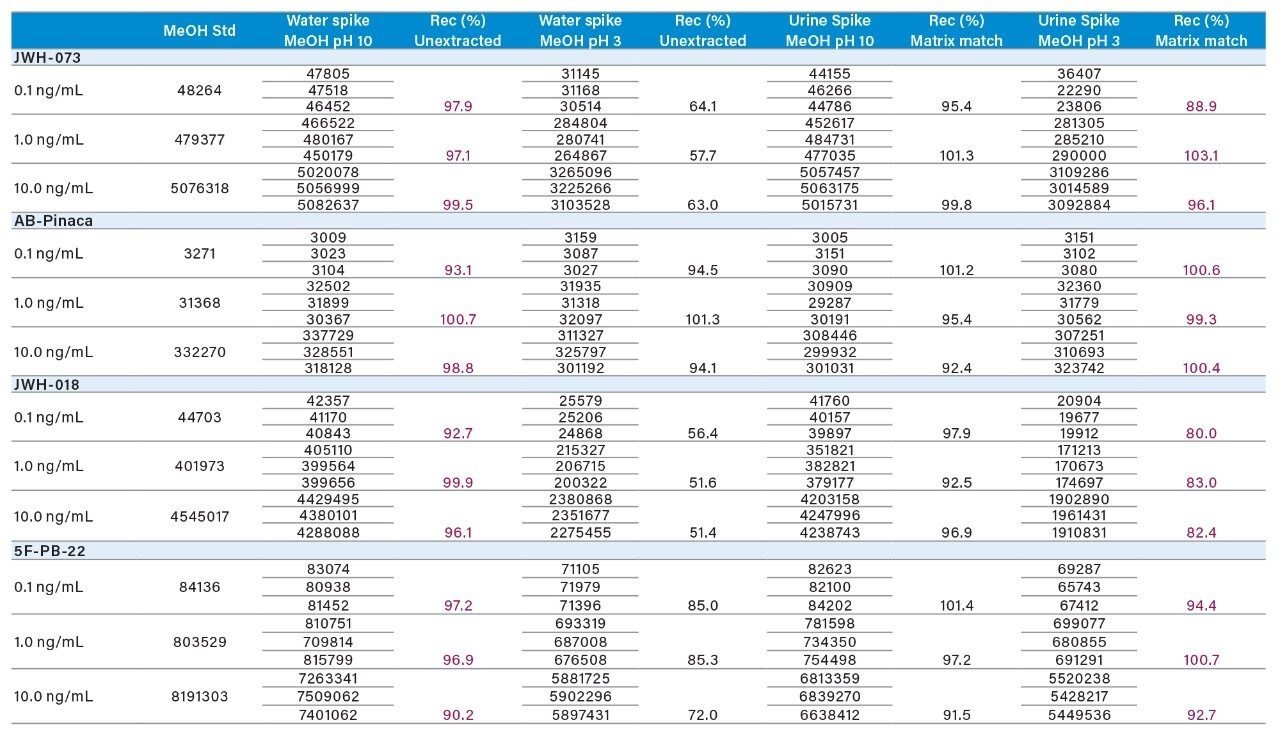
For the urine spike, the low area counts in comparison to their aqueous spikes indicate strong matrix effects, predominantly suppression effects. One observation worth mentioning is the complete reversal of elution conditions. The results suggest for the urine spiked sample, a low-pH methanol elution yielded better recoveries for all analytes (>75% range), except for erythromycin and clindamycin at 50% and 30%, respectively. The area counts for urine and water spiked samples with low-pH methanol elution gave similar values.
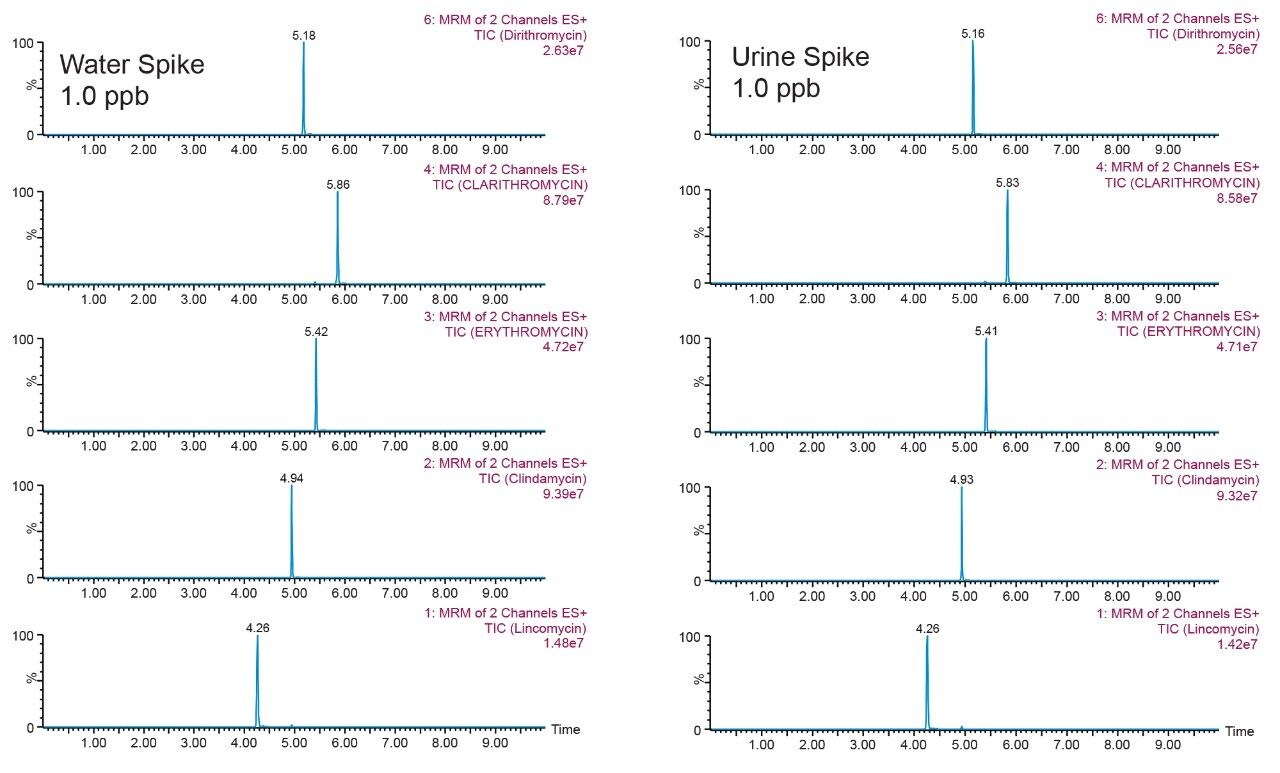
The extraction protocol used in this work was designed for a generic screening approach, meaning that the wash step was as mild as possible so not to elute any crucial analyte (target or unknown) during the wash step. In this instance, the only wash step was a simple 5% methanol wash between the loading phase and elution phase. For intermediate and complex samples, the drawback of a single wash extraction protocol will be an increased signal on the background noise, usually visible by extra peaks and baseline distortion at the expected retention time of a target analyte. Here the urine extract showed a clean baseline at 1 ppb with an intense signal, suggesting the feasibility of a low pertrillion range detection (Figure 14). As for the cannabinoids, only AB-pinaca showed extra peaks and baseline distortion; a mild case and far away from the target analyte, which suggests that at the expected retention time of AB-pinaca there is no visible interferences (Figure 15).
Overall, the use of a 2D-LC-MS/MS method made it possible to produce a successful, five-day method for the analysis of macrolides and cannabinoids. The workflow started with the infusion of the target analyte at three pH values to determine which pH would provide the best signal. The quick 3 × 3 LC-MS/MS overnight runs gave a clear chromatography map and made it possible to have a better understanding of the analytes’ chromatographic behavior. Once the LC method was chosen, most of the evaluation time was focused on the optimization of the extraction protocol. For the cannabinoids, optimal LC conditions were found to include a C18 trap column with a pH 3 loading, and a C18 analytical column with an acetonitrile elution at pH 10 (Method 11). The SPE elution with acetonitrile at pH 3 yielded satisfactory results. The limit of detection was identified to be 0.1 ng/mL; however, for the macrolides, the optimal LC conditions included an HLB trap column with a pH 3 loading, and a C18 analytical column with an acetonitrile elution at pH 3 (Method 3). The SPE elution with methanol at pH 3 gave excellent results. The limit of detection was identified to be 0.1 ng/mL.
720006741, January 2020